Diabetes mellitus represents a rising public health concern and is a major health challenge in Oman and in other countries globally.1-3 The impact of this non-communicable disease — in terms of its diagnosis and management by different levels of health care providers — has made measurement of glucose among the most commonly requested core biochemistry test worldwide both as laboratory-based or point-of-care testing. Accurate measurement of glucose in blood, plasma, or serum is required for the diagnosis, treatment and assessment of diabetes mellitus. Hence, the use of defined “cut-off” values of glucose is required with arrangements for ensuring adherence to proper pre-analytical requirements of all variables in the process.4
In 1941 sodium fluoride (NaF) containing tubes were introduced into laboratory practice for blood collection for glucose measurement.5 NaF has been known to have an antiglycolytic effect that inhibits glycolysis by erythrocytes. The use of these tubes appeared to be suitable for blood collection when laboratory practice was not streamlined and there was a long delay in blood separation following collection, a practice that continued, and was not questioned or revisited, for more than five decades.
Taking into consideration that NaF action in inhibiting enolase enzyme in glycolytic pathway is slow, as it will start after nearly four hours from blood collection, its effect has recently been critically questioned.6,7 The most recent guidelines in 2011 for the diagnosis and management of diabetes mellitus approved by the American Diabetes Association (ADA) no longer recommends the use of NaF to control glycolysis, with the recommendation to stop its use.8 Gambino9 criticized the false belief that NaF is an effective inhibitor of glycolysis. Bruns10 also questioned whether fluoride-containing tubes are still needed for blood glucose testing in his editorial support of the study by Fernandez et al,11 which reported no difference in glucose values for blood specimens collected in serum separator tubes (SST) and NaF tubes that were separated within two hours of collection.
The objectives of this study were to compare glucose concentrations measured in plasma -NaF tubes and serum -SST tubes analyzed in a hospital setting, and to assess the pattern of change in glucose concentrations in both tubes from the time of receipt in the laboratory and up to seven days after collection.
Methods
This study was conducted at the Clinical Biochemistry Laboratory, Royal Hospital, Oman, during a three month period (1 September–30 November 2013). Blood specimens were processed using the same laboratory setting for specimens handling and processing. During the study period, the group of blood specimens that were received in the laboratory for patients for whom two types of blood tubes were collected (one NaF for lactate and one SST for core biochemistry or immunology tests) were included in this study. Following separation of the plasma -NaF and serum -SST, glucose concentrations were measured in both plasma and serum by an enzymatic hexokinase assay (assay CV ≤5%), using Architect c8000 with automated processing system (Abbott, USA). Following completion of the analytical measurement of glucose, random selection of 15 pairs of NaF and SST tubes containing the separated plasma and serum, respectively, were kept in the refrigerator at 4oC and glucose concentrations were measured daily up to seven days after the receipt of the blood containing tubes. In our laboratory the blood collection-to-separation time is usually up to one hour.
Results
Comparing plasma -NaF and serum -SST results of glucose values (n=50, paired t-test) showed no significant difference. The mean ± standard deviation (SD) glucose concentrations were 9.80 ±7.94mmol/L (NaF) versus 9.80 ±7.91mmol/L (SST) with an average difference of 0.00mmol/L (range -0.60 to +0.60mmol/L). Of the 50 plasma/serum pairs compared, 20 (40%) showed similar values while 30 (60%) showed a small difference in glucose values (median 0.00mmol/L, range -0.60 to + 0.60mmol/L). Bland Altman analysis [Figure 1] gave a non-significant constant bias of 0.10 ±0.195mmol/L (bias ±SD). Pearson correlation [Figure 2] between plasma -NaF and serum -SST glucose concentrations revealed a highly significant correlation approaching unity with r2= 0.9991 (y= 0.9914x + 0.017) [Figure 2]. In addition, the difference in glucose concentration in each of 15 pairs containing plasma -NaF and serum SST showed no significant difference between the time of receipt and seven days post-receipt; [Figures 3 and 4]. Hemolysis was observed in five (10%) NaF tubes compared with two (4%) SST tubes.
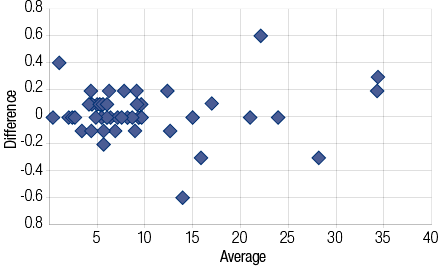
Figure 1: Bland Altman plot for the difference in glucose measurement with NaF tubes and SST tubes.
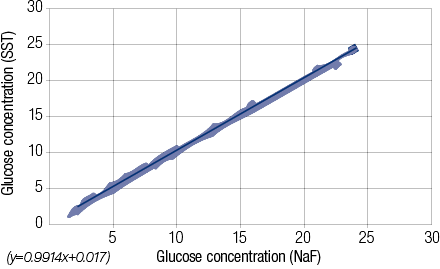
Figure 2: Pearson correlation of glucose concentrations measures from NaF tubes and serum SST tubes.
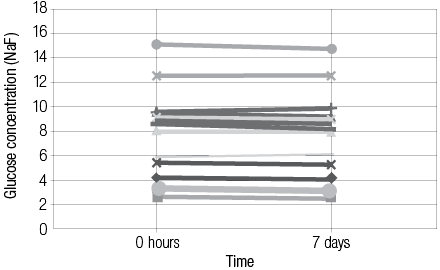
Figure 3: The difference in glucose concentration collected in plasma (NaF tubes) at 0 hour and 7 days.
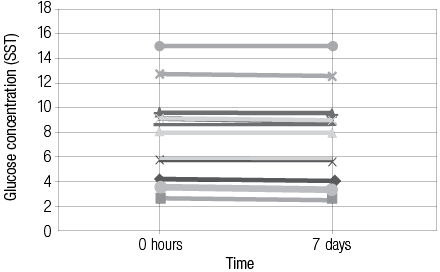
Figure 4: The difference in mesured glucose concentration collected in blood SST tubes at 0 hour and 7 days.
Discussion
Our study confirmed that both SST and NaF tube can be used to collect blood for glucose measurement with similar reported glucose values under routine practice in a hospital laboratory setting. There was no significant difference in glucose concentrations in plasma and serum from both types of tubes given that blood sample processing in our hospital setting has an optimum collection-to-separation of less than one hour. Also, following serum or plasma separation, glucose concentration was stable during the storage period at 4oC for seven days (which is the time studied) or longer (although this was not tested). Less hemolysis was noted in SST (4%) compared with NaF tubes (10%). These results are comparable to the findings by Fernandez et al,11 and Li et al12 who found glucose measurement in SST and heparinized tubes similar to NaF tubes.
While some studies reported small but significant bias towards lower glucose concentrations in samples collected in NaF tubes, other studies reported higher glucose values in NaF tubes, the difference reported was proportional to the time delay in blood separation.13-16 The explanation for the discrepancy is that studies that reported lower glucose results in NaF tubes were separated shortly or immediately after blood collection. However, lower glucose values in tubes not containing NaF were associated with blood specimens with more than four hours delay in separation. Bruns7 recently viewed this issue critically comparing the current and previous reports in this field, some of which are his own studies, with his support for the use of SST in preference to NaF tubes for laboratory based glucose measurement in the current practice. Bruns10 in his editorial in 2013 valued his earlier finding in the 1980s of concordant glucose concentrations for tubes with and without NaF in his study in a university hospital routine practice, which was not fully explained at that time.
The progress and improvement in the speed of blood tubes processing, adoption of lean techniques in laboratory practice, improved logistics of specimens transported to the laboratory (with implementation of pneumatic tubing system in some hospitals) and improved quality of tubes including the wide-spread use of SST and similar tubes, such as heparinized tubes, have culminated in collection-separation times of less than one hour. Glucose measurement in plasma or serum can be done without the need for NaF if blood separation is achieved within reasonable time (less than two hours). Longer delay should be avoided not only for the purpose of glycolysis preventation but also for the quality of other analytes including certain electrolytes, enzymes, and hormones that are known to be affected by delay in blood separation. The issue, which includes the disadvantage of NaF tubes use has been strengthened by the 2011 ADA laboratory guidelines for the diagnosis and management of diabetes mellitus with its recommendation to stop NaF use as antiglycolysis agent.8 The ADA guidelines also recommended blood separation within 30 minutes. In case a delay in blood separation is expected, the use of acid citrate is suggested as an immediate antiglycolytic agent. However, these tubes have been recently described, are not broadly available, and their use might require revisiting of the glucose cut-off thresholds for the diagnosis of diabetes mellitus, recommended by the ADA, World Health Organization, and other professional bodies, which may not easily and practically be considered for implementation.17,18
Finally, for serum or plasma glucose measurement the use of SST tubes in preference to NaF tubes can offer many specimen processing advantages as these universal tubes can be used for the majority of biochemistry and immunology tests reducing the need for other consumables and the amount of blood drawn from the patients, and improving turn-around-time and laboratory workflow. Despite the low cost of NaF tubes, and bearing in mind the large number of laboratory based glucose tests performed annually, significant financial savings can be made when these tubes are replaced by SST, which are commonly used for a variety of laboratory tests with the same single collection tube used for a panel of tests, including glucose.
Conclusion
There was no difference in glucose values in plasma and serum collected in NaF collection and SST, respectively, when serum separation was achieved within reasonable time of less than one to two hours following blood collection. Glucose concentrations are stable for many days in both separated tubes when stored refrigerated, which is common in hospital laboratories. Hence, for blood glucose measurement in laboratory practice in hospital setting the commonly used blood collection tubes (SST and heparinized tubes) are universal collection tubes for many tests. Also, when these tubes are used for glucose measurement there will be practical advantages including cost-effectiveness and reduction in blood drawn when utilizing these tubes for different laboratory tests, including glucose, from a single blood collection tube. However, if blood separation is delayed or expected to be delayed for more than two hours following blood collection, then fluoride containing tubes can be used for glucose measurement.
Disclosure
The authors declared no conflict of interests. No funding was received for this work.
references
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011 Jul;378(9785):31-40.
- Al Riyami A, Elaty MA, Morsi M, Al Kharusi H, Al Shukaily W, Jaju S. Oman world health survey: part 1 - methodology, sociodemographic profile and epidemiology of non-communicable diseases in oman. Oman Med J 2012 Sep;27(5):425-443.
- Al-Sinani S, Al-Shafaee M, Al-Mamari A, Woodhouse N, Al-Shafie O, Hassan M, et al. Familial clustering of type 2 diabetes among Omanis. Oman Med J 2014 Jan;29(1):51-54.
- Engelgau MM, Thompson TJ, Herman WH, Boyle JP, Aubert RE, Kenny SJ, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care 1997 May;20(5):785-791.
- Bueding E, Goldfarb W. The effect of sodium fluoride and sodium iodoacetate on glycolysis in human blood. J Biol Chem 1941;14:539-544.
- Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem 2007 Dec;53(12):2040-2041.
- Mikesh LM, Bruns DE. Stabilization of glucose in blood specimens: mechanism of delay in fluoride inhibition of glycolysis. Clin Chem 2008 May;54(5):930-932.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011 Jun;57(6):e1-e47.
- Gambino R. Sodium fluoride: an ineffective inhibitor of glycolysis. Ann Clin Biochem 2013 Jan;50(Pt 1):3-5.
- Bruns DE. Are fluoride-containing blood tubes still needed for glucose testing? Clin Biochem 2013 Mar;46(4-5):289-290.
- Fernandez L, Jee P, Klein MJ, Fischer P, Perkins SL, Brooks SP. A comparison of glucose concentration in paired specimens collected in serum separator and fluoride/potassium oxalate blood collection tubes under survey ‘field’ conditions. Clin Biochem 2013 Mar;46(4-5):285-288.
- Li G, Cabanero M, Wang Z, Wang H, Huang T, Alexis H, et al. Comparison of glucose determinations on blood samples collected in three types of tubes. Ann Clin Lab Sci 2013;43(3):278-284.
- Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem 1989 Feb;35(2):315-317.
- Waring WS, Evans LE, Kirkpatrick CT. Glycolysis inhibitors negatively bias blood glucose measurements: potential impact on the reported prevalence of diabetes mellitus. J Clin Pathol 2007 Jul;60(7):820-823.
- Shi RZ, Seeley ES, Bowen R, Faix JD. Rapid blood separation is superior to fluoride for preventing in vitro reductions in measured blood glucose concentration. J Clin Pathol 2009 Aug;62(8):752-753.
- Turchiano M, Nguyen C, Fierman A, Lifshitz M, Convit A. Impact of blood sample collection and processing methods on glucose levels in community outreach studies. J Environ Public Health. 2013; 2013:256151.
- Gambino R, Piscitelli J, Ackattupathil TA, Theriault JL, Andrin RD, Sanfilippo ML, et al. Acidification of blood is superior to sodium fluoride alone as an inhibitor of glycolysis. Clin Chem 2009 May;55(5):1019-1021.
- Bruns DE, Knowler WC. Stabilization of glucose in blood samples: why it matters. Clin Chem 2009 May;55(5):850-852.