| |
Abstract
Objectives: Malnutrition is prevalent among cancer patients, and maybe correlated with altered quality of life. The objective of this study is to determine whether quality of life among cancer patients on chemotherapy at the National Kidney and Transplant Institute- Cancer Unit differs from patients with normal nutrition based on the Subjective Global Assessment scale.
Methods: A cross sectional study was conducted among cancer patients admitted for chemotherapy at the National Kidney and Transplant Institute-Cancer Unit from January to May 2011. Demographic profile, performance status by Eastern Cooperative Oncology Group performance scale, nutritional status assessment by Subjective Global Assessment, and quality of life assessment by the European Organization for Research and Treatment of Cancer QoL-30 core module were obtained. Descriptive statistics and ANOVA were performed for analysis of quality of life parameters and nutritional status.
Results: A total of 97 subjects were included in this study, 66 subjects (68.04%) were females and 31 (31.96%) were males. Mean age was 54.55 ± 11.14 years, while mean performance status by the Eastern Cooperative Oncology Group classification was 0.88 ± 0.83 with a range of 0-3. According to the Subjective Global Assessment, there were 58 patients with SGA A, classified to have adequate nutrition, and 39 patients (40.21%) were considered malnourished. Among these 39 patients, 32 were classified SGA-B (moderately malnourished) and 7 were classified SGA C (severely malnourished) mean global quality of life was 68.73 ± 19.05. Results from ANOVA test revealed that patients were statistically different across the Subjective Global Assessment groups according to global quality of life (p<0.001), physical (p<0.001), role (p<0.001), emotional (p<0.001), and cognitive functioning (p<0.001); fatigue (p<0.001), nausea and vomiting (p<0.001), pain (p<0.001), insomnia (p<0.001), and appetite loss (p<0.001).
Conclusion: Global quality of life and its parameters: physical state, role, emotional state, cognitive functioning, cancer fatigue, nausea and vomiting, pain, insomnia, and loss of appetite were statistically different across all Subjective Global Assessment groups. Moreover, there was no difference between financial difficulties, social functioning, constipation and diarrhea among the Subjective Global Assessment groups.
Keywords: Cancer nutrition; Quality of life; Subjective global assessment.
Introduction
Coeliac disease (CD), formerly named nontropical sprue or coeliac sprue (from the Dutch word sprue) is an immune-mediated intolerance to gluten (from wheat, barley, or rye) in genetically susceptible individuals.1,2 Although patients with CD were previously known to be presented with severe diarrhea, malabsorption, stomatitis and weight loss; a wide range of clinical manifestations of systemic involvements however, may develop during the course of the disease.3 Also, although CD was originally considered a disease of infants and children with malabsorption, it is now considered to affect all ages including the elderly.4
The diagnostic criteria for CD are usually based on the histological changes in jejunal biopsies of patients, while they are on and off gluten diet as per guidelines of the European Society of Pediatric Gastroenterology and Nutrition (ESPGAN). The characteristic findings include intraepithelial lymphocytosis, crypts hyperplasia and villous atrophy with improved response to gluten free diet.5 From 1990, the ESPGAN criteria added value on the finding of positive circulating antibodies (IgA gliadin, antireticulin and antiendomysium) at the time of diagnosis and their disappearance together with the improvement of symptoms when the patients are on gluten free diet.6,7 The sensitivity of serological tests, particularly antiendomysium or antitissue transglutaminase antibodies is greater than 90%, and tests for either markers is considered to be the best means for CD screening.8 The National Institute of Clinical Excellence (NICE) has also recently recommended the use of serological testing for these antibodies as a first-line test for patients with suspected CD.9
Due to the availability of such accurate and practical non-invasive serological tests for CD, as well as increased medical awareness about its existence, CD has shifted from what was thought to be a rare disease to a common one. During the last two decades, there has been an increase in the reported prevalence of CD, particularly in the Caucasian population with a prevalence of around 1% in the UK and European populations.10-12 The high prevalence is also attributed to the inclusion of Marsh I-II in the definition of CD, often termed as gluten-sensitive enteropathy (GSE).
This study aims to estimate the prevalence of CD in at-risk subjects, describe the clinical characteristics and laboratory findings associated with CD and the validity of serological testing for CD at the Royal Hospital, Oman during a period of three years.
Methods
This retrospective audit case finding study was conducted at a tertiary care hospital, the Royal Hospital in Muscat, Oman. Ethical approval for conducting this work was obtained from the Research and Ethical Review Committee, Directorate of Research and Studies, Royal Hospital, on 10/4/2012.
The medical and laboratory records of the patients for whom serum antiendomysium IgA antibodies (EMA) test, a serological marker of CD, was requested during the three years period (1st Jan 2006-31st Dec 2008) were reviewed. The results of EMA were compared with the results of histology of the jejunal biopsies (when available). The laboratory, clinical and demographic characteristics were extracted for all the patients for whom investigations for CD were considered, in order to assess the followings:
a. Prevalence rate of CD among at-risk subjects
b. Clinical characteristics including main manifestations of CD, presence of other associated diseases, and clinical manifestations for which the requesting clinicians considered CD as a possible diagnosis, including the specialty of the requesting clinician.
c. Laboratory tests result particularly, in relation to the biochemical and hematological indicators of anemia, vitamin deficiency, liver function and bone profile.
d. Validity indicators of EMA testing in comparison with histopathology of jejunal biopsies for diagnosing or excluding CD.
The diagnosis of CD was made on the basis of results of jejunal biopsy histopathology examination and EMA testing, as well as response to gluten-free diet. At the time of study, serum specimens for EMA assay were referred to Biomnis Laboratory, France, (EMA positive cut-off at titre >10 IU/L). The jejunal histopathological examinations were done at the Histopathology Laboratory, Royal Hospital. The features consistent with CD included: hyperplasia of crypts, atrophy of villous, and increase of intraepithelial lymphocytes.
The data collected included patient demographics, presenting symptoms, other associated clinical manifestations or diseases, and specialty of the requesting clinicians. The results of the laboratory tests (where available) included: CBC (including mainly: hemoglobin concentration, MCV, MCH, RDW), ferritin, transferrin saturation, vitamin B12, folate, bone profile, liver function test, total IgA, EMA and jejunal histopathology.
The data were analysed using SPSS statistical software. Descriptive statistical data were presented to summarise the study variables as number, percentage, mean ± SD or median (range) as appropriate. The prevalence rate of CD among at-risk subjects was calculated by dividing the number of patients with proven CD during the study period by the population size in whom the disease was screened. The validity indicators (sensitivity, specificity, positive predictive value, negative predictive value and efficiency) were calculated for the test (EMA) in comparison with the gold standard (biopsy).
Results
During the three years study period (1st Jan 2006-31st Dec 2008), EMA testing was requested for 431 patients who were suspected by the requesting clinician of having CD. There were 250 females and 181 males, with median age of 15 years (mean ± SD: 18.95 ± 14.1 years) and a range of 9 months-74 years. Of these, 15 (3.5%) patients (10 females, 5 males) had positive EMA with concentrations of 204.5 ± 160 IU/L and median (range) of 160 (40-320) IU/L. The median age in these 15 patients was 19 years and mean 21.4 ± 13.0 years (range: 2.5-38 years). Of these 15 patients, 13 patients had jejunal biopsy which all showed positive histopathological changes indicative of CD. The remaining 2 patients had no biopsy examination; both had type 1 diabetes mellitus (DM) and were aged 9 months and 28 years with EMA results of 80 and 70 IU/L, respectively. Forty-four patients with negative EMA <10 IU/L had jejunal biopsy, of whom 41 patients were negative for CD changes and 3 patients had histopathological changes (Marsh I), suggestive of mild CD. All the 3 patients had serum total IgA levels within the reference range with results of 3.0, 2.4 and 3.5 g/L (NR 0.5-3.7 g/L). Taking these results into consideration and that the two patients with negative EMA and Marsh I changes as being GSE or possibly early CD, the calculated validity indicators of EMA for the diagnosis of CD are: sensitivity 81.3%, specificity 100%, positive predictive value 100%, negative predictive value 93.2% and efficiency 94.7%.
In the 15 patients with CD, the most common mode of presentation was gastrointestinal, accounting for 7/15 patients and comprising of diarrhea (6), abdominal pain (4), weight loss (1): followed by type 1 DM (5/15), clinical anemia (5/15), short stature or failure to thrive (4/15) and hypothyroidism (2/15), one patient had both type 1 DM and hypothyroidism, Table 1. Screening for CD is usually considered in the work-up assessment for type 1 diabetics, and in this study, 102 patients were screened for CD and 5 were found to be EMA seropositive making the prevalence of EMA seropositivity indicative of CD in type 1 diabetics to be 4.9% in Oman.
Table 1: Main Clinical manifestations at presentation in patients with Coeliac Disease (n= 15).
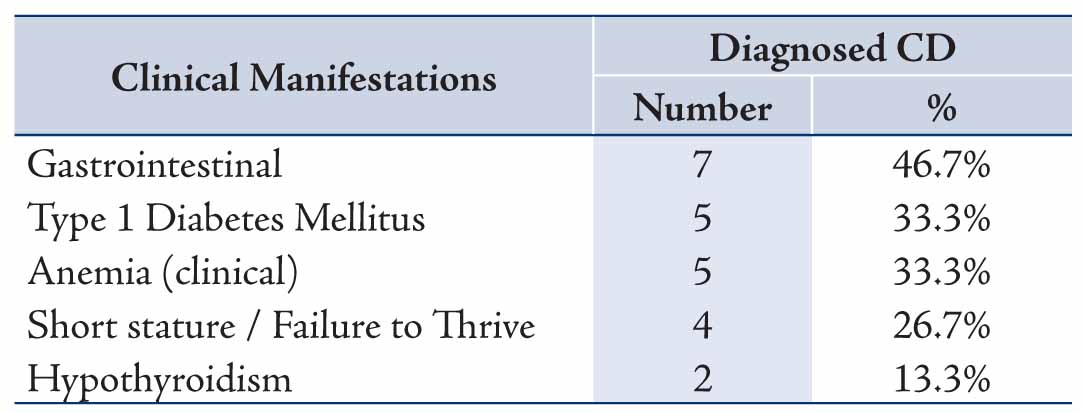
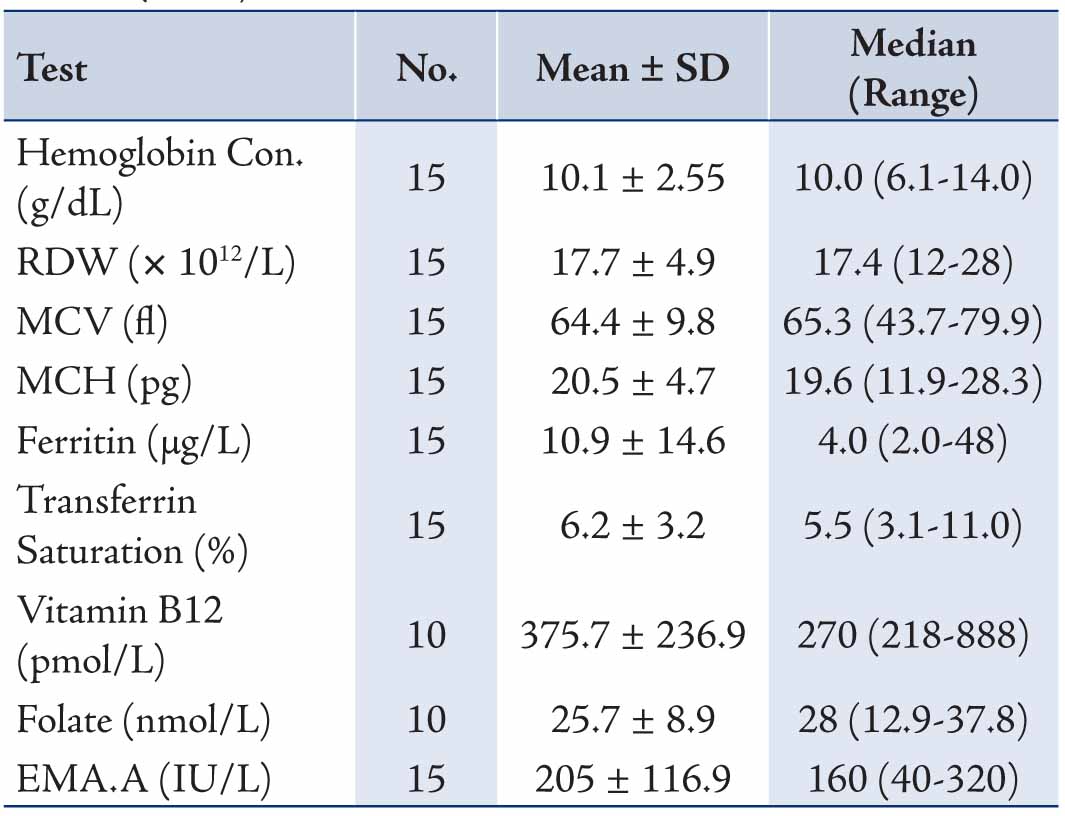
For the clinical manifestations for which diagnostic suspicion of CD was considered, screening for CD in type 1 DM was the commonest indication for requesting EMA, followed by the presence of gastrointestinal symptoms, short stature, anemia and thyroid disease, Table 2. For the specialties of the clinicians requesting EMA, the test was most frequently requested by both pediatric and adult endocrinologists (53.8%) and similarly both pediatric and adult gastroenterologists (40.6) with less being requested by other physicians, surgeons and gynecologists (all 5.6%), Table 3. The results of the different laboratory tests for the 15 patients with CD are presented in Table 4. Twelve patients showed low ferritin (<12 μg/L in females, <18 μg/L in males) and low transferrin saturation (<10%) indicative of iron deficiency anemia.
Table 2: Clinical presentation and diagnosis of 431 patients for whom serum EMA testing was requested.
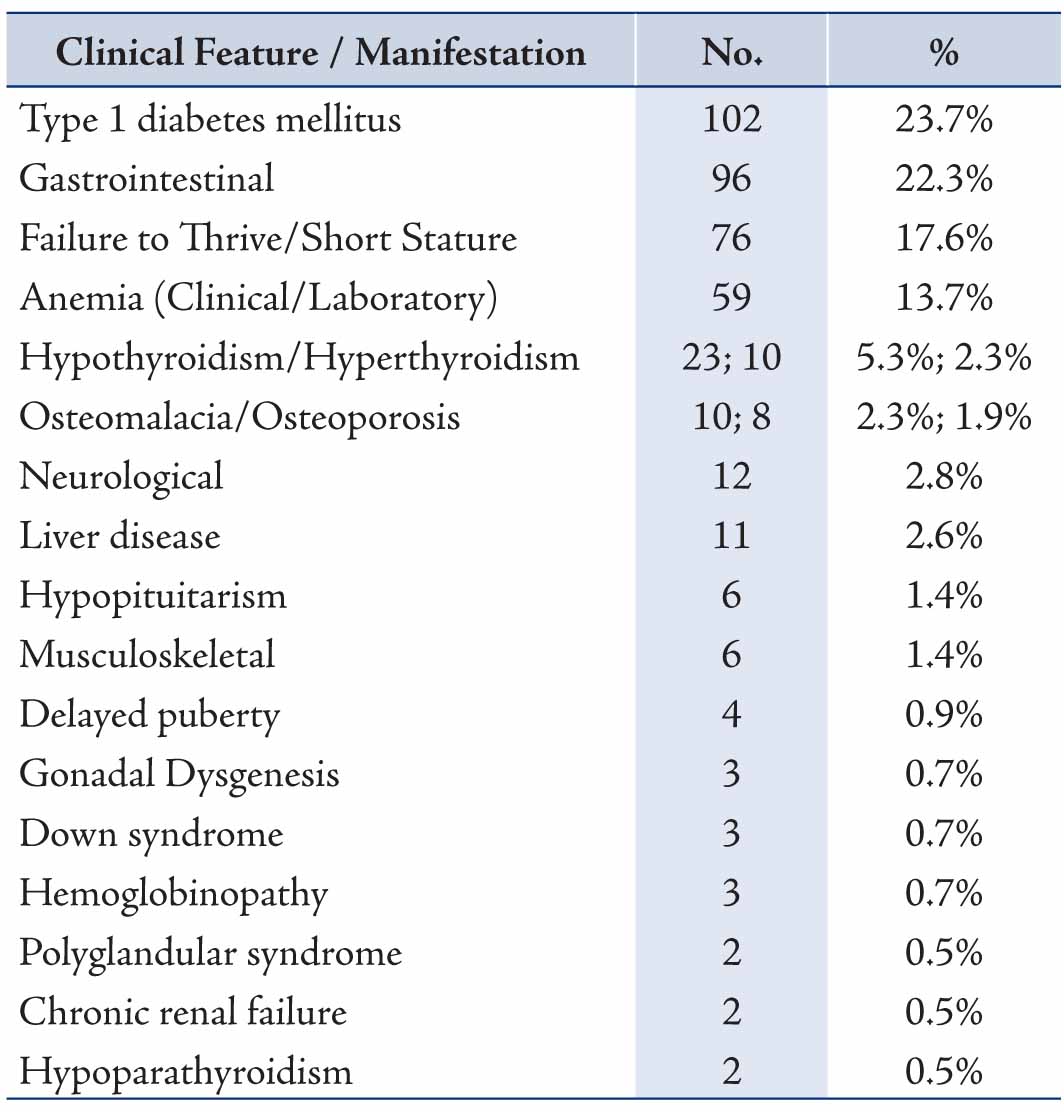
Table 3: Specialty of clinicians who requested serum EMA for the 431 patients .
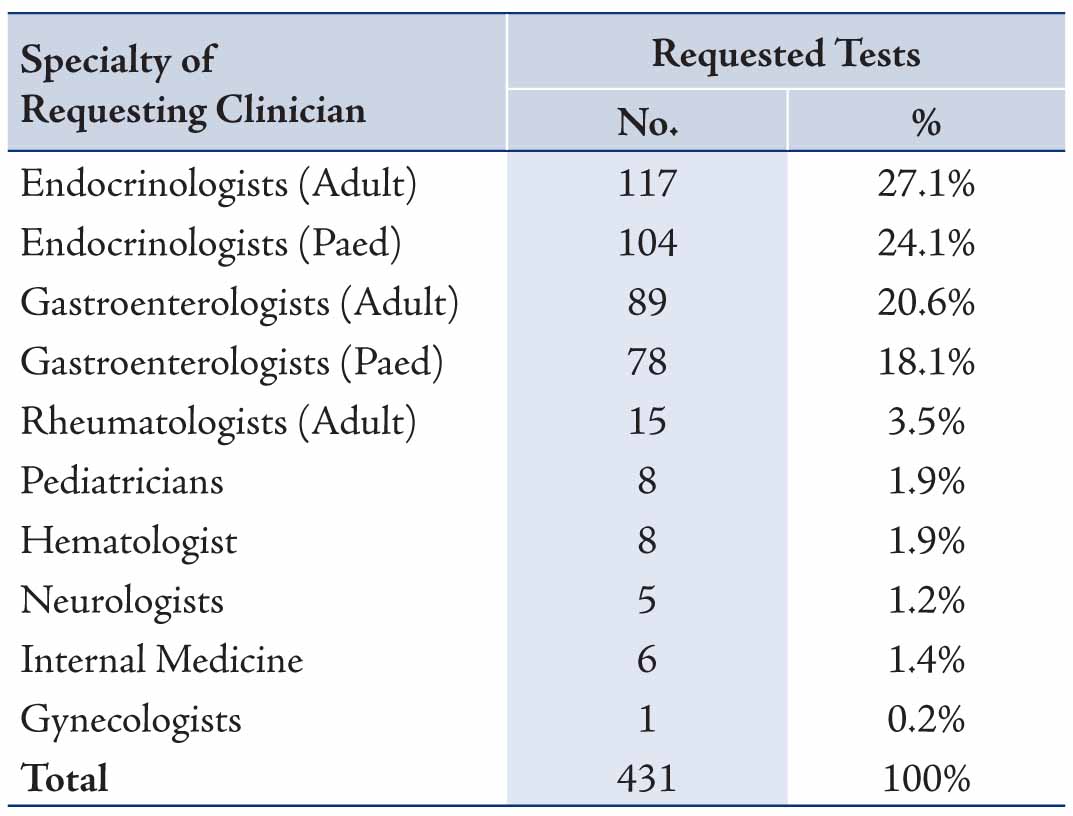
Table 4: Results of laboratory tests for patients with Coeliac Disease (n=15).
Discussion
This study revealed that 15 cases (10 females, 5 males) were diagnosed to have CD from a total of 431 patients who were investigated for this disease at the Royal Hospital, Oman during a period of three years (2006-2008). Giving a prevalence rate for CD of 3.5% that was reported for at-risk patients with manifestations or diseases suggestive to be present or associated with CD. The median (range) of age for the patients with CD was 19 years (2.5-38 years) and for patients who underwent EMA testing was 15 years (9 months-74 years). The broad age range reflects the clinicians’ awareness about CD occurrence among all age groups and not only children as what was previously thought for CD as being a childhood disease.13 It also appears that CD prevalence is increasing in our region with figures approaching those in developed countries.
In comparison with other studies, Hin et al.11 reported 30 cases of CD among 1000 high-risk hospital patients, giving a CD prevalence of 3%, which is comparable to the prevalence of 3.5% observed in the current case finding study. In Eastern Saudi Arabia, in clinically CD suspected patients, the seropositivity was 7.6% with histology confirmed CD at 4%.14 Also, the prevalence of CD in a healthy adult Saudi population who volunteered in a prospective study at a blood donation centre in Jeddah, Saudi Arabia was 1.5%.15 This prevalence rate seems to be approaching the high rates reported in Caucasian populations.10-12,16 Concerning age, the age range of patients with CD in a series by Sanders et al12 was 1-82 years. It has been recognized that about 25% of patients with CD are diagnosed in patients above the age of 60 years.17 Few studies related to CD have been conducted in Oman. Fraser et al.18 reported a 3.3% prevalence rate of CD among 51 patients with unexplained iron deficiency anemia. While Akinbami et al.19 reported CD in 13% of 62 children presented with chronic diarrhea in their 7 year prospective study. Also, recently Al-Lawati et al.20 reported 9 children with CD with mean age of 7.1 years at diagnosis in their 6 year search at Royal Hospital.
Coeliac disease is an immune-mediated disorder that may affect different organs in the body with a variety of clinical and laboratory manifestations. Our study observed a wide spectrum of clinical presentations that might be considered to be as a result of CD by the requesting clinicians. These clinicians ordered EMA with further consideration of jejunal biopsy examination for patients with different clinical features including gastrointestinal, endocrinological, hematological, musculoskeletal, as well as other signs and symptoms. It was interesting to note that the majority of EMA requests were made by the endocrinologists (adult 27.1%, pediatric 24.1%) and by gastroenterologists (adult 20.6%, pediatric 18.1%). This points to the increasing awareness among the endocrinologists, besides the gastroenterologists for considering CD in the differential diagnosis, including the work-up investigations in type 1 DM. However, the requesting rate is still less for the other specialties, since much less requests were made by rheumatologists (3.5%), pediatricians (1.9%), hematologists (1.9%), internists (1.4%), neurologists (1.2%) and gynecologists (0.2%). No request was made by other clinicians including Surgeons.
In our study, the clinical manifestations (in decreasing order) in the fifteen patients with CD were gastrointestinal, type 1 DM, anemia, short stature or/and failure to thrive and hypothyroidism. Iron deficiency anemia was evident in 80% of our CD cases as evidenced from serum ferritin and trasferrin saturation as well as hematological indices (hemoglobin concentration, MCV, MCH, RDW). In their review of 264 patients with CD Sender et al.12 found that 28.4% of patients had gastrointestinal symptoms, 20.1% had iron deficiency anemia, and 52.7% were diagnosed by gastroenterologists. Hin et al.11 in their 30 cases of CD who were diagnosed during screening 1000 high-risk subjects at nine clinics using EMA in 1997 found that 15/30 patients had anaemia and 25/30 had non-gastrointestinal symptoms. Also in our study, the small number of cases might have limited the reporting of other features which are expected to be found in CD. In addition, screening for CD is part of the work-up investigations for patients with type 1 DM, and of the 102 type 1 diabetics who were screened for CD in our study, 5 (4.9%) were EMA seropositive. The prevalence rate of CD in type 1 DM varies in the literature with a range of 1-19% (mean 4.5%) as reviewed in 26 reports by Holmes.21
Our study revealed high predictive values for serum EMA in diagnosing CD with sensitivity 81.3%, specificity 100%, positive predictive value 100%, negative predictive value 93.2% and efficiency 94.7%. There was a very good agreement with the histological findings. Serological tests have been used as surrogate markers of CD for more than 20 years with an increasing interest in their use in the diagnosis of CD.22 The IgA class EMA and anti-tissue transglutaminase (anti-tTG) ELISA assays are considered to be reliable tests for diagnosing CD.8,20 Patients with negative serology (in the absence of IgA deficiency) and gluten-dependent enteropathy together with minimal histological changes are considered to have CD-like enteropathy (or GSE).8,23-25 When IgA deficiency exists, serological testing using IgG class EMA and anti-tTG are recommended to be measured. IgA deficiency, defined as serum IgA <0.1 g/L may occur at a prevalence of one in approximately 500, being less common in Asian population with variable reports of its occurrence in patients with CD of one in 39 patients in one study and one in 131 in another study.26-29 Screening or testing for CD may therefore aid the detection or diagnosis of IgA deficiency or hypogammaglobulinemia.30 No patient with IgA deficiency was reported in our patient series in this study. In patients with IgA deficiency, testing for CD IgG EMA and anti-tTG serology is recommended. Recently, newer assays incorporating synthetic deamidated gliadin-related peptide (DGPs) or other tTG isoenzymes or antigens have been described.31,32 These assays are available as kits for combined detection of IgA- and IgG- class antibodies to DGP (DGP dual) and for IgA and IgG antibodies to both tTG and DPG (tTG/DGP screen). These tTG/DGP screen and DGP Dual assays may represent useful tools to confirm gluten sensitivity in IgA anti tTG-seronegative patients (whether IgA deficient or not) who are suspected to have CD. However, further experience in their utilization is required before justifying their practical advantages.32-35 Currently in our laboratory, testing for IgA class anti-tTG and total IgA is performed as a CD screening panel. This in-house test availability, together with increased awareness of CD following the introduction of new guidelines in 2009 on the indications of CD testing,9 has resulted in increased requests at our laboratory for serological screening of CD.
Our study has some limitations, namely; The study reported the prevalence of CD among at-risk subjects with symptoms or diseases suspected of the disease. Assessment of prevalence of CD among healthy subjects such as blood donors or those contributing in screening programs is recommended. In addition, the serological diagnosis was based only on IgA class EMA. It is worth to study the validity of other serological tests which have been used as surrogate markers of CD such as anti-tTG and DGPs, including their combined detection of IgA- and IgG- class antibodies (tTG/DGP screen and DGP dual). Also, longitudinal studies for the prevalence of CD among patients with metabolic associates of CD such as diabetes mellitus, iron deficiency anemia, osteoporosis, and failure to thrive is worth much consideration.
Conclusion
The availability of serological tests and increased medical awareness has increased requests for the diagnosis of CD. The results showed that serum antiendomysium IgA antibodies is a highly sensitive and specific test for diagnosing CD and that the diagnosis is made mainly by the endocrinologists and gastroenterologists (pediatric or adult), but to a lesser extent by other clinicians. Furthermore, the disease was fount to be associated mainly with anemia, gastrointestinal features, type 1 diabetes mellitus, failure to thrive or hypothyroidism and serum EMA.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Leeds JS, Hopper AD, Sanders DS. Coeliac disease. Br Med Bull 2008;88(1):157-170.
2. Green PH, Cellier C. Celiac disease. N Engl J Med 2007 Oct;357(17):1731-1743.
3. Collins JR, Isselbacher KJ. Treatment of adult celiac disease (nontropical sprue). N Engl J Med 1964 Nov;271(22):1153-1156.
4. Stavropoulos SN, Panagi SG, Goldstein SL, Mcmahon DJ, Absan H, Neugut AI; Green PHR. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol 2001 Jan;96(1):126-131.
5. Walker-Smith DH, Guandalini S, Schmitz J, Shmerling DH, Visakorpi JK. Revised criteria for diagnosis of celiac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child 1990;65(8):907-911.
6. Johnston SD, Watson RG, McMillan SA, Sloan J, Love AH. Prevalence of coeliac disease in Northern Ireland. Lancet 1997 Nov;350(9088):1370.
7. Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 2003 Feb;163(3):286-292.
8. Rostom A, Dube C, Cranney A, Saloojee N, Sy R, Garritty C, et al. The diagnostic accuracy of serologic tests for celiac disease: a systemic review. Gastrenterology 2005;128(Suppl 1):S38-S46 .
9. Coeliac disease: Recognition and assessment of celiac disease; NICE Clinical guideline 86, May 2009. www.nice.org.uk/
10. van Heel DA, West J. Recent advances in coeliac disease. Gut 2006 Jul;55(7):1037-1046.
11. Hin H, Bird G, Fisher P, Mahy N, Jewell D. Coeliac disease in primary care: case finding study. BMJ 1999 Jan;318(7177):164-167.
12. Sanders DS, Hurlstone DP, Stokes RO, Rashid F, Milford-Ward A, Hadjivassiliou M, et al. Changing face of adult coeliac disease: experience of a single university hospital in South Yorkshire. Postgrad Med J 2002 Jan;78(915):31-33.
13. Zipser RD, Farid M, Baisch D, Patel B, Patel D. Physician awareness of celiac disease: a need for further education. J Gen Intern Med 2005 Jul;20(7):644-646.
14. Al Attas RA. How common is celiac disease in Eastern Saudi Arabia? Ann Saudi Med 2002 Sep-Nov;22(5-6):315-319.
15. Khayyat YM. Serologic markers of gluten sensitivity in a healthy population from the western region of Saudi Arabia. Saudi J Gastroenterol 2012 Jan-Feb;18(1):23-25.
16. Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systemic review. Gastrenterology 2005;128(4)(Suppl 1):S57-S67 .
17. Holmes G, Catassi C. Clinical manifestations. In: Holmes G, Catassi C, Eds. Coeliac Disease, Oxford: Health Press, 2000; p32-3.
18. Fraser JS, Woodhouse NJ, El-Shafie OT, Al-Kindy SS, Ciclitira PJ. Occult celiac disease in adult Omanis with unexplained iron deficiency anemia. Saudi Med J 2003 Jul;24(7):791.
19. Akinbami FO, Venugopalan P, Elnour IB, Nirmala V, Abiodun P, Azubuike JC. Pattern of chronic diarrhoea in children: a prospective analysis of causes, clinical features and outcome. Niger Postgrad Med J 2006 Mar;13(1):53-56.
20. Al-Lawati TT, Al-Musawi HS. Celiac disease in oman: a tertiary centre experience. Oman Med J 2013 Jan;28(1):70-72.
21. Holmes GK. Screening for coeliac disease in type 1 diabetes. Arch Dis Child 2002 Dec;87(6):495-498.
22. Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992 Jan;102(1):330-354.
23. Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology 2005 Apr;128(4)(Suppl 1):S25-S32.
24. Tesei N, Sugai E, Vázquez H, Smecuol E, Niveloni S, Mazure R, et al. Antibodies to human recombinant tissue transglutaminase may detect coeliac disease patients undiagnosed by endomysial antibodies. Aliment Pharmacol Ther 2003 Jun;17(11):1415-1423.
25. Naiyer AJ, Hernandez L, Ciaccio EJ, Papadakis K, Manavalan JS, Bhagat G, et al. Comparison of commercially available serologic kits for the detection of celiac disease. J Clin Gastroenterol 2009 Mar;43(3):225-232.
26. Latiff AH, Kerr MA. The clinical significance of immunoglobulin A deficiency. Ann Clin Biochem 2007 Mar;44(Pt 2):131-139.
27. Holt PD, Tandy NP, Anstee DJ. Screening of blood donors for IgA deficiency: a study of the donor population of south-west England. J Clin Pathol 1977 Nov;30(11):1007-1010.
28. Wang N, Shen N, Vyse TJ, Anand V, Gunnarson I, Sturfelt G, et al. Selective IgA deficiency in autoimmune diseases. Mol Med 2011;17(11-12):1383-1396.
29. McGowan KE, Lyon ME, Butzner JD. Celiac disease and IgA deficiency: complications of serological testing approaches encountered in the clinic. Clin Chem 2008 Jul;54(7):1203-1209.
30. Sugai E, Vázquez H, Nachman F, Moreno ML, Mazure R, Smecuol E, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol 2006 Sep;4(9):1112-1117.
31. Bright P, Lock RJ, Unsworth DJ. Immunoglobulin A deficiency on serological coeliac screening: an opportunity for early diagnosis of hypogammaglobulinaemia. Ann Clin Biochem 2012 Sep;49(Pt 5):503-504.
32. Niveloni S, Sugai E, Cabanne A, Vazquez H, Argonz J, Smecuol E, et al. Antibodies against synthetic deamidated gliadin peptides as predictors of celiac disease: prospective assessment in an adult population with a high pretest probability of disease. Clin Chem 2007 Dec;53(12):2186-2192.
33. Agardh D. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin Gastroenterol Hepatol 2007 Nov;5(11):1276-1281.
34. Sugai E, Hwang HJ, Vázquez H, Smecuol E, Niveloni S, Mazure R, et al. New serology assays can detect gluten sensitivity among enteropathy patients seronegative for anti-tissue transglutaminase. Clin Chem 2010 Apr;56(4):661-665.
35. Villalta D, Tonutti E, Prause C, Koletzko S, Uhlig HH, Vermeersch P, et al. IgG antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem 2010 Mar;56(3):464-468.
|
|