|
Abstract
Objectives: H. pylori infection has been associated with some autoimmune disorders. The aim of this study was to evaluate the serum concentrations of rheumatoid factor and anti-nuclear antibodies in H. pylori-infected peptic ulcer patients, H. pylori-infected asymptomatic carriers and a healthy control group.
Methods: A Total of 100 H. pylori-infected peptic ulcer patients, 65 asymptomatic carriers and 30 healthy H. pylori-negative subjects (as a control group) were enrolled into study. Serum samples of participants tested for the levels of rheumatoid factor and anti-nuclear antibodies by use of ELISA.
Results: The mean serum levels of rheumatoid factor and anti-nuclear antibodies in peptic ulcer group was significantly higher in comparison to the control group (p<0.05). Although, the mean serum levels of rheumatoid factor and anti-nuclear antibodies in the asymptomatic carriers group was higher than those in the control group, the difference was not statistically significant. No significant differences were observed between peptic ulcer patients and asymptomatic carriers groups regarding the mean serum levels of rheumatoid factor and anti-nuclear antibodies. The mean serum levels of rheumatoid factor in men with peptic ulcer was significantly higher compared to the group of healthy men (p<0.05). Although in female of peptic ulcer patients or asymptomatic carriers groups, the mean serum levels of rheumatoid factor was higher than that in healthy women, but the differences were not statistically significant. Also, no significant differences were observed between men and women with peptic ulcer, asymptomatic carriers control groups based on the serum levels of anti-nuclear antibodies.
Conclusion: The results showed higher serum levels of rheumatoid factor and anti-nuclear antibodies in H. pylori-infected patients with peptic ulcer disease which represent the H. pylori-related immune disturbance in these patients. Additional follow-up studies are necessary to clarify the clinical significance of these autoantibodies in patients with H. pylori infection.
Keywords: Helicobacter pylori; Peptic ulcer; Rheumatoid factor; Anti-nuclear antibody.
Introduction
Helicobacter pylori (H. pylori) is one of the most frequent chronic bacterial infections in humans and has been associated with several gastroduoedenal diseases, including gastric ulcers, duodenal ulcers, gastric mucosa-associated lymphoid tissue lymphomas and gastric cancer.1 Gastric colonization with H. pylori causes peptic ulcer (PU) in 10-20% and gastric cancer in less than 3% of those infected.2 The interplay of interactions between bacterial virulence parameters and host genetic factor determine the outcome of infection.1,3 We have previously reported that the seroprevalence of H. pylori in healthy Iranian adults and children was 67.5% and 46.6%, respectively.4
H. pylori infection causes not only a variety of gastroduodenal diseases, but is also involved in the pathogenesis of various autoimmune disorders such as rheumatoid arthritis (RA), idiopathic thrombocytopenic purpura, Sjogren's syndrome, autoimmune gastric atrophy, anti-phospholipid antibody syndrome, autoimmune thyroiditis and Henoch-Schoenlein purpura.5,6 It has also recently been reported that a connection between H. pylori infection and the occurrence of anti- thyroid peroxidase (anti-TPO), anti-thyroglobulin autoantibodies and autoimmune thyroiditis in young patients with type 1 diabetes.7
Rheumatoid factor (RF) is an antibody recognizing the Fc portion of human antibodies, and is present in 60%-90% of RA patients. Chronically high titers of RF are thought to be more specific for RA as well as being prognostic of poor outcomes.8 The autoantibody RF may exist in subjects many years before they exhibit RA and its appearance awards a risk of developing RA that increases with rising titer.9
The production of autoantibodies against nuclear antigens such as anti-nuclear antibodies (ANA) is the hallmark of systemic lupus erythematosus (SLE).10 It should be noted that the association of the H. pylori infection with ANA has not been studied adequately.
H. pylori infection has also been associated not only with chronic gastritis and peptic ulcer, but also with many extra-intestinal disorders. Among the latter, a correlation has been found between this bacterium and various autoimmune diseases. So far, the potential role of H. pylori infection in RA has been poorly investigated. In this study, we evaluated the serum levels of RF and ANA in H. pylori-infected PU patients, H. pylori-infected asymptomatic (AS) carriers as well as in healthy uninfected subjects to determine whether there is a possible link between the bacterium and these autoantibodies.
Methods
Totally, 100 H. pylori-infected PU patients (age: 38.70 ± 12.94 years), 60 H. pylori-infected asymptomatic (AS) subjects (age: 38.36 ± 11.99 years) and 30 uninfected (H. pylori-negative) healthy subjects (age: 37.90 ± 10.48 years) were included in the study.
The PU patients had the disease confirmed by upper gastrointestinal endoscopy and none of the subjects were taking medications (including nonsteroidal anti-inflammatory drugs) at the time of study. In all patients, the H. pylori status was determined by the rapid urease test and testing for the presence of serum H. pylori-specific immunoglobulin G (IgG) antibodies with commercial enzyme-linked immunosorbent assay (ELISA) kits. The rapid urease test (RUT) was performed on a gastric antrum biopsy specimen that was obtained during endoscopic examination. The patients were considered positive for H. pylori infection if both tests (RUT and serological H. pylori-specific IgG) were simultaneously positive.
The AS carriers were positive for H. pylori infection by serological screening of anti-H. pylori-specific IgG antibodies. The AS carriers, as well as the uninfected healthy subjects were recruited from among blood donors and were interviewed with regard to gastrointestinal manifestations (e.g., dyspepsia), and none had any history of gastrointestinal or any other relevant diseases. Moreover, individuals with past medical history of cardiovascular disease, hypertension, diabetes mellitus, pulmonary disease, asthma, renal failure, anemia, neoplasia, autoimmune diseases (such as RA and SLE) and those on drug were excluded from the study. AS and healthy controls did not undergo endoscopy and were basically healthy, with no acute or chronic sickness. A blood sample was taken from all participants and the sera were separated and stored at -70°C until analysis.
This study was evaluated and approved by the Ethical Committee of Rafsanjan University of Medical Sciences. Moreover, patients were recruited only if they gave consent for blood sampling.
The serum levels of anti-H. pylori immunoglobulin G were measured using the commercial enzyme-linked immunosorbent assay (Trinity Biotec, Ireland). Briefly, 100 µl of control samples and serum samples (in duplicates) were pipetted into the microtitre wells, which were coated with H. pylori specific immunodominant antigens. The plates were incubated for 30 min at 25°C. H. pylori- specific antibody, if present, binds to the antigen forming antigen-antibody complexes. The wells were washed 5 times with washing solution to remove non-band antibodies. Then, 100 µl of antihuman IgG conjugated with hours radish peroxidase was pipetted into each well, and the plates were incubated for 30 min at 25°C again. At this stage, the conjugate binds to the antibody-antigen complexes. The wells were then washed 5 times with washing solution to remove excess conjugate. A fresh substrate solution, tetramethylbenzidine (100 µl) was added, and the plates were incubated for 15 min at room temperature. If specific antibody to the antigen is present in the patient's serum, a blue color develops which is proportional to the concentration of anti-H. pylori antibodies in the serum. The enzyme reaction was stopped with 100 µl of 1 N H2SO4. The absorbance of each well was determined at 450 nm with a spectrophotometer ELISA microplate reader. According to manufacturer guidlines the results were expressed as Immune Status Ratio (ISR), the ISR values of ≥1.1 were considered to positive and the sensitivity of the method was estimated to be 100%.
Serum RF levels were measured by the turbidimetry method using commercial kits (Biosystem, Barcellona, Spain). RF causes the agglutination of the latex particles coated with human gammaglobulin. The agglutination of the latex particles is proportional to the RF concentration and is measured by turbidimetry. The serum levels of RF were quantitated by using standard samples with known concentration of factor expressed as IU/mL, provided by the manufacturer.
The serum levels of ANA were also measured through the commercial ELISA kits (Genesis, Cambridgeshine, UK). The ELISA kit contains microplates. The wells of microplates were coated with antigen. In the first step, the serum samples are incubated in the wells. After washing, the anti-human IgG conjugated with an enzyme is added to wells. After incubation, the unbound conjugate is removed by washing and then the enzyme substrate is added. After color development, the optical density of the samples is measured using a microplate reader. The optical density is directly proportional to the antibody concentration in the sample. The serum levels of ANA were quantitated using standard samples with known concentrations of antibodies, provided by the manufacturers.
Statistical analysis was done using the computer program (SPSS, version 15, chicago, IL, USA). Differences between the variables were analyzed using Student t-test, ANOVA, Mann-Whitney U and Kruskal-Wallis as appropriate and p values of less than 0.05 were considered significant.
Results
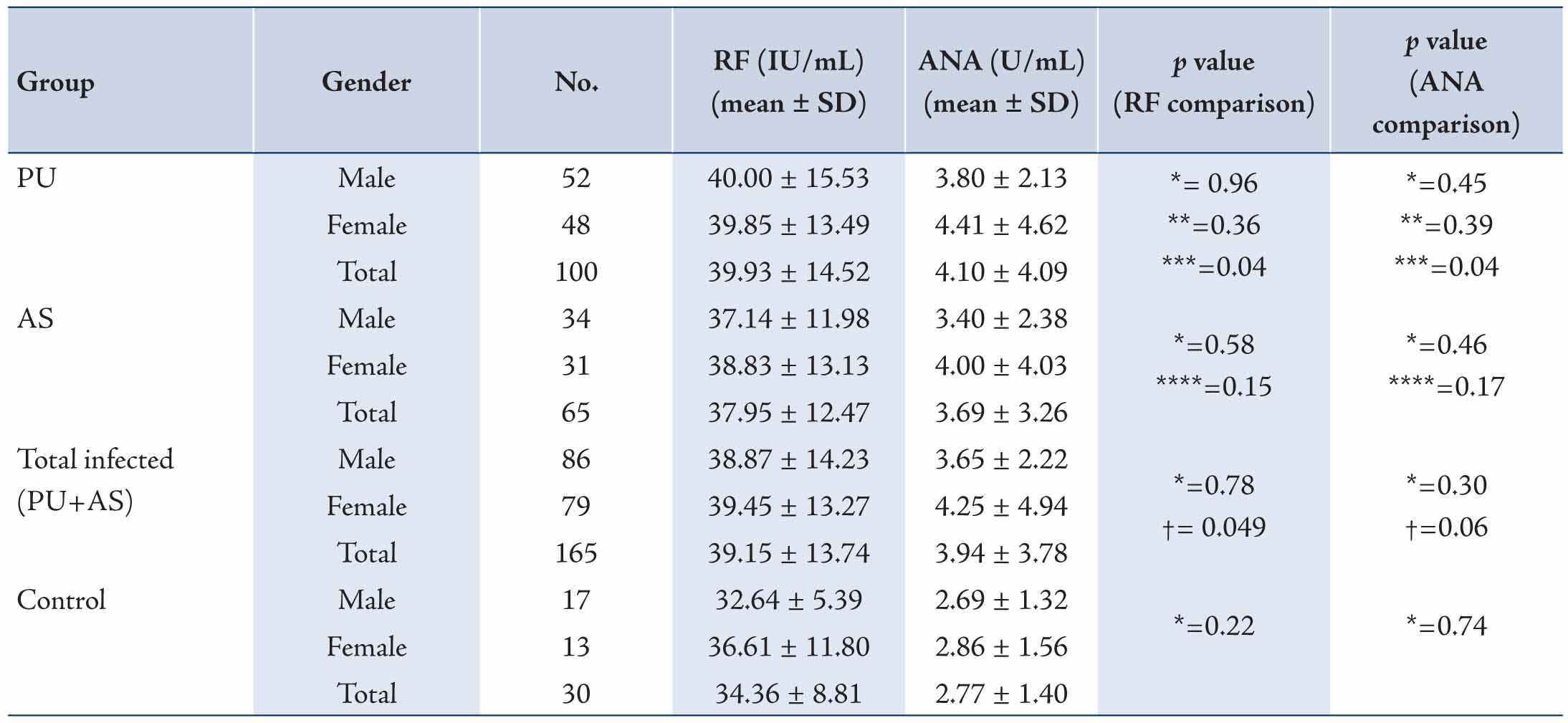
The mean serum levels for RF and ANA in the healthy controls and patient groups are demonstrated in Table 1. The mean serum levels of RF in the PU group was significantly higher (39.93 ± 14.52 IU/mL) than that observed in the control group (34.36 ± 8.81 IU/mL; p<0.05). Although the mean serum levels of RF in the AS group was higher (37.95 ± 12.47 IU/mL) than that in control group, the difference was not statistically significant. Moreover, no significant difference was observed in terms of the mean serum levels of RF between the PU and AS groups. While the mean serum levels of RF among the total H. pylori-infected subjects (PU patients plus AS subjects) was also significantly higher in comparison to uninfected control group (p<0.05).
Also, the mean serum levels of autoantibodies according to the gender of participants are also shown in Table 1. In the PU, AS and control groups, no significant differences were observed between men and women in terms of the mean serum levels of RF. However, the mean serum levels of RF in men with PU was significantly higher (40.00 ± 15.53 IU/mL) compared to the group of healthy men (32.64 ± 5.39 IU/mL; p<0.05). Although in women of PU or AS groups, the mean serum levels of RF were higher than in contrast with the group of healthy women, but the differences were not statistically significant.
As demonstrated in Table 1, the mean serum levels of ANA was significantly higher in the PU grouped compare to the control group (4.10 ± 4.09 U/mL vs. 2.77 ± 1.40 U/mL; p<0.05). While the mean serum levels of ANA in the AS group (3.69 ± 3.26 IU/mL) was also higher than that in the control group, but the difference did not reach statistically significance. The difference serum levels of ANA between the PU and AS groups was also not statistically significant. Thus, no significant differences were also observed between men and women of PU, AS and control groups based on the serum levels of ANA (Table 1). In addition, the mean serum levels of ANA in total H. pylori-infected subjects (PU patients plus AS subjects) was higher than the levels for the uninfected control group, but the difference was not statistically significant (p=0.06).
Table 1: A Comparison of levels of serum rheumatoid factor and anti-nuclear antibodies between PU, AS and control groups according to gender.
Discussion
The results of the present study showed higher serum levels of RF and ANA in H. pylori-infected PU patients compared to the healthy control group. In accordance with our results, it has been reported that the H. pylori infection contributes in the pathogenesis of rheumatoid arthritis, and the eradication at the infection is reported to have reduced the disease severity.11,12 Moreover, the eradication of H. pylori is recommended for infected RA patients in order to attenuate the disease.11 It has been also reported that the eradication of H. pylori reduces several laboratory parameters including ANA, erythrocyte sedimentation rate, fibrinogen and α2-globulins in patients with RA.11
The possible mechanisms responsible for the elevation of RF and ANA levels in H. pylori-infected PU patients still remains to be determined. Some H. pylori components may be responsible for this phenomenon. It has been demonstrated that both cell surface-bound urease and purified urease isolated from H. pylori activate murine B cells to produce various autoantibodies, such as immunoglobulin M (IgM)-type RF, anti-single-stranded DNA and antiphosphatidylcholine antibodies.13 Moreover, it has also been reported that the direct interaction of extracellular toll like receptors (TLR2) on B-1 type cells with H. pylori urease induces the production of various autoantibodies in a T-cell-independent manner.14 This may account for the association of various autoimmune diseases with H. pylori infection. Accordingly, it has been suggested that H. pylori components such as urease may be among the environmental inducers that initiate several autoimmune diseases by inducing the production of autoantibodies via the activation of B-1 cells.13
It has also been reported that immune responses to H. pylori infection, through molecular mimicry mechanisms may contribute to several extragastric disorders.15 Accordingly, the cross mimicry mechanism between microorganism and host antigens such as Fc portion of IgG and nuclear antigens may also account for the production of autoantibodies following H. pylori infection.
Since the H. pylori express several immunodominant antigens, detection of serum antibodies to some of these antigens would be a reliable marker for the diagnosis of H. pylori infection.16 Serological tests are non-invasive methods of value for the diagnosis of H. pylori infection because they are simple and convenient.17 The most frequently used serological test for H. pylori infection is ELISA due to its simplicity, low cost and high sensitivity, and it has also been reported to be highly accurate for the diagnosis of H. pylori infection in adults.18 We used an ELISA commercial kit for the diagnosis of H. pylori infection. The sensitivity of ELISA kit in this study has been previously reported to be 100% for Iranian population.19 In the present study, the H. pylori infection was determined by RUT in PU patients, and the presence of H. pylori-specific IgG using ELISA. The PU patients were considered positive for H. pylori infection if both tests were simultaneously positive. However, for AS and healthy control groups, the H. pylori infection was determined based on the presence of H. pylori-specific IgG. Due to the invasive nature of RUT test, we could not obtain the biopsy sample from AS and control groups due to ethical restrictions. However, it has been reported that the results of serological methods are comparable with biopsy based RUT for the diagnosis of H.pylori infection.19
The results here also demonstrated that RF and ANA levels were significantly higher than the control group only in the PU group, but the differences of RF and ANA levels between AS and control group were not significant. The possible mechanisms responsible for higher levels of ANA and RF in the PU patients are still unclear. The results of this study represent that H. pylori infection may solely partly contribute to the induction of autoantibody production. Indeed, the development of PU disease markedly increases the titer of autoantibodies. In other word, the rise in serum autoantibodies levels may mostly be as a result to the immunopathologic responses that occur during PU disease. In PU, there is severe tissue damage and inflammation responses which cause the release of normally sequestered autoantigens and the subsequent activation of autoreactive lymphocytes, as well as the production of autoantibodies. The released autoantigens from gastrointestinal tissues may mimic other self antigens such as nuclear antigens and Fc portion of IgG. On the other hand, virulence factors of H. pylori such as CagA can also influence the clinical outcome of the infection. Genetically, H. pylori is divided into CagA-positive and CagA-negative strains and it has been reported that CagA+ strains are associated with more severe inflammatory reactions and an increased risk of adverse clinical outcomes in western countries.20 In our previous study, the seroprevalence of CagA+ strains was significantly higher in PU patients in comparison to AS subjects.21 Accordingly, higher rates of infection with CagA+ strains may also directly and/or indirectly induce higher titers of RF and ANA in PU patients.
It should be noted that the pathogenesis of autoimmune diseases such as RA and SLE is multifactorial, involving both genetic and environmental factors (including bacterial and viral agents).22 In a particular genetic background, H. pylori infection may be considered as a etiologic factor for the expression of some autoimmune disease. In other word, H. pylori infection may not be exclusively a cause for the induction of autoimmune responses, but rather, the coexistence of a certain genetic parameters is necessary for development of autoimmunity. It has also been demonstrated that the genetic background such as major histocompatibility complex (MHC) alleles influence the development of autoimmune diseases including RA and SLE. Significant associations have been reported between SLE and RA with HLA-DR3 and HLA-DR4 alleles, respectively.23,24 It has also been reported that the co-presence of H. pylori and HLA-DRB1*0301 allele may contribute to the development of autoimmune thyroid disease.25 Similarly, the coexistence of H. pylori and HLA-DR3/DR4 alleles may specifically be involved in the induction of immune responses against self-antigens such as ANA and RF production in H. pylori-infected subjects. In other words in subjects possessing the HLA-DR3/DR4 alleles, H. pylori infection may preferentially activate an irregular immune response that causes RF and ANA production.
The decrease of antithyroid antibodies titer has been observed after eradication of H.pylori infection.26 Similarly, H. pylori eradication may decrease RF and ANA titers and prevent the development of related diseases. These findings suggest that there is an opportunity for attempting to eradicate H. pylori in order to prevent or attenuate related diseases in H. pylori-infected subjects. However, additional follow-up studies are also necessary to clarify the clinical significance of RF and ANA in patients with H. pylori infection. It should be noted that the results indicating the clinical improvement of RA by H. pylori eradication are controversial.11,12,27 Immunologically, TNF-α has been reported as a proinflammatory cytokine that plays a key role in the pathogenesis of RA.28 Patients with H. pylori infection have been reported to up-regulate TNF-α and its receptor.29 Thus, H. pylori eradication may result in an amelioration of RA activity via the down-regulation of TNF-α. Moreover, in immune system, the helper T(TH)-lymphocyte cells are divided into at least three distinct subsets, i.e., TH1, TH2, and TH17 cells that are characterized by distinct cytokine release profiles and cell/cytokine functions. It has been proven that RA is a TH1-dependent disease.30 In H. pylori-infected patients; up-regulation of TH1 responses have also been demonstrated.30 Accordingly, H. pylori eradication may result in clinical improvement of RA. However, other infectious agents apart from H. pylori may also participate in the development of RA.27 Therefore, H. pylori eradication may not result in clinical improvement of RA in all cases.
Our results showed that the mean serum levels of RF in men with PU was significantly higher compared to the group of healthy men. Although in female patients the mean serum levels of RF was higher than in the control group, but the differences were not statistically significant. These results suggest an association between RF and PU in men. The differential PU pathogenesis and/or differential hormonal pattern may account for these observations. Despite similar prevalence of H. pylori between men and women, most studies show the male gender predominance for the development of peptic ulcer, therefore men have a higher prevalence of H. pylori-associated ulcer than women.31,32 Moreover, it has been shown that men have a higher gastric acid secretory capacity than women which may be a reason for the differential expression of gender-related peptic ulcer.33 Other possible mechanisms include the protection of women against duodenal ulceration by oestrogens via the induction of bicarbonate secretion by epithelial cells.34 In an animal experimental model, it has been demonstrated that major male (testosterone) and female (progesterone) sex hormones exhibit opposite effect on healing of ulcers in the oral cavity and stomach, so that testosterone markedly delays while progesterone significantly accelerates ulcer healing.35 In some epidemiological studies higher seroprevalence of CagA+ strains has also been reported in males.36 Altogether, these findings indicate that the H. pylori infection may induce more severe tissue damage and stronger inflammatory responses in men compared to women, which may account for higher levels of ANA and RF in men than women.
It should be noted that this study may have had its limitations: (i) the measurement of autoantibodies was done at one time only, thus the findings could not provide information as to when the antibodies were elevated and for how long the elevation of antibodies continued. Antibodies may have been also formed during immunopathological responses after the PU event; (ii) the effects of circulating autoantibodies on long-term clinical outcomes were not part of the protocol. Estimating the time course of autoantibodies during the PU disease might introduce a prognostic value; the results of our study encourage further studies into this field; (iii) measurement of autoantibodies was performed on samples that were stored at -20°C, therefore we cannot exclude the possibility of protein degradation. However, this would have affected both cases and controls in a similar manner.
Conclusion
The results of the present study indicate that there are higher serum levels of RF and ANA in PU patients. It further suggests that the gender of patients may influence the production of autoantibodies.
Acknowledgements
The authors are grateful to Mohammad Ahmad-Beaygi and the authorities of the endoscopy unit at Rafsanjan Ali-ebn- Abitaleb Hospital for their invaluable help.
References
1. Backert S, Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter 2011 Sep;16(Suppl 1):19-25.
2. Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006 Jul;19(3):449-490.
3. Snaith A, El-Omar EM. Helicobacter pylori: host genetics and disease outcomes. Expert Rev Gastroenterol Hepatol 2008 Aug;2(4):577-585.
4. Jafarzadeh A, Rezayati MT, Nemati M. Specific serum immunoglobulin G to H pylori and CagA in healthy children and adults (south-east of Iran). World J Gastroenterol 2007 Jun;13(22):3117-3121.
5. Ohta M. [Helicobacter pylori infection and autoimmune disease such as immune thrombocytopenic purpura]. Kansenshogaku Zasshi 2010 Jan;84(1):1-8.
6. Hasni S, Ippolito A, Illei GG. Helicobacter pylori and autoimmune diseases. Oral Dis 2011 Oct;17(7):621-627.
7. El-Eshmawy MM, El-Hawary AK, Abdel Gawad SS, El-Baiomy AA. Helicobacter pylori infection might be responsible for the interconnection between type 1 diabetes and autoimmune thyroiditis. Diabetol Metab Syndr 2011;3(1):28.
8. Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis 2011; 2011:815038.
9. Dörner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Curr Opin Rheumatol 2004 May;16(3):246-253.
10. Ardoin SP, Pisetsky DS. Developments in the scientific understanding of lupus. Arthritis Res Ther 2008;10(5):218.
11. Zentilin P, Seriolo B, Dulbecco P, Caratto E, Iiritano E, Fasciolo D, et al. Eradication of Helicobacter pylori may reduce disease severity in rheumatoid arthritis. Aliment Pharmacol Ther 2002 Jul;16(7):1291-1299.
12. Zentilin P, Garnero A, Tessieri L, Dulbecco P, Seriolo B, Rovida S, et al. [Can Helicobacter pylori infection be a risk factor for the severity of rheumatoid arthritis?]. Recenti Prog Med 2000 Apr;91(4):175-180.
13. Yamanishi S, Iizumi T, Watanabe E, Shimizu M, Kamiya S, Nagata K, et al. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. Infect Immun 2006 Jan;74(1):248-256.
14. Kobayashi F, Watanabe E, Nakagawa Y, Yamanishi S, Norose Y, Fukunaga Y, et al. Production of autoantibodies by murine B-1a cells stimulated with Helicobacter pylori urease through toll-like receptor 2 signaling. Infect Immun 2011 Dec;79(12):4791-4801.
15. Gasbarrini G, Racco S, Franceschi F, Miele L, Cammarota G, Grieco A, et al. [Helicobacter pylori infection: from gastric to systemic disease]. Recenti Prog Med 2010 Jan;101(1):27-33.
16. Chomvarin C, Ottiwet O, Hahnvajanawong C, Intapan PM, Wongwajana S. Seroreactivity to specific antigens of Helicobacter pylori infection is associated with an increased risk of the dyspeptic gastrointestinal diseases. Int J Infect Dis 2009 Sep;13(5):647-654.
17. Alsaimary I, Al-Sadoon M, Jassim A, Hamadi S. Clinical findings and prevalence of helicobacter pylori in patients with gastritis B in Al-basrah governorate. Oman Med J 2009 Jul;24(3):208-211.
18. Leal YA, Flores LL, García-Cortés LB, Cedillo-Rivera R, Torres J. Antibody-based detection tests for the diagnosis of Helicobacter pylori infection in children: a meta-analysis. PLoS One 2008;3(11):e3751.
19. Mohammadi M, Talebkhan Y, Khalili G, Mahboudi F, Massarrat S, Zamaninia L, et al. Advantage of using a home-made ELISA kit for detection of Helicobacter pylori infection over commercially imported kits. Indian J Med Microbiol 2008 Apr-Jun;26(2):127-131.
20. Costa AC, Figueiredo C, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter 2009 Sep;14(Suppl 1):15-20.
21. Jafarzadeh A, Salari M. Seroprevalence of anti-Helicobacter pylori and anti-CagA antibodies in peptic ulcer and healthy subjects in the city of Rafsanjan. J Res Med Sci 2006;11(5):285-291.
22. Invernizzi P, Gershwin ME. The genetics of human autoimmune disease. J Autoimmun 2009 Nov-Dec;33(3-4):290-299.
23. Castaño-Rodríguez N, Diaz-Gallo LM, Pineda-Tamayo R, Rojas-Villarraga A, Anaya JM. Meta-analysis of HLA-DRB1 and HLA-DQB1 polymorphisms in Latin American patients with systemic lupus erythematosus. Autoimmun Rev 2008 Feb;7(4):322-330.
24. Charpin C, Balandraud N, Guis S, Roudier C, Toussirot E, Rak J, et al. HLA-DRB1*0404 is strongly associated with high titers of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis. Clin Exp Rheumatol 2008 Jul-Aug;26(4):627-631.
25. Larizza D, Calcaterra V, Martinetti M, Negrini R, De Silvestri A, Cisternino M, et al. Helicobacter pylori infection and autoimmune thyroid disease in young patients: the disadvantage of carrying the human leukocyte antigen-DRB1*0301 allele. J Clin Endocrinol Metab 2006 Jan;91(1):176-179.
26. Bertalot G, Montresor G, Tampieri M, Spasiano A, Pedroni M, Milanesi B, et al. Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin Endocrinol (Oxf) 2004 Nov;61(5):650-652.
27. Matsukawa Y, Asai Y, Kitamura N, Sawada S, Kurosaka H. Exacerbation of rheumatoid arthritis following Helicobacter pylori eradication: disruption of established oral tolerance against heat shock protein? Med Hypotheses 2005;64(1):41-43.
28. Voulgari PV. Golimumab: a new anti-TNF-alpha agent for rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Expert Rev Clin Immunol 2010 Sep;6(5):721-733.
29. Zhao C, Lu X, Bu X, Zhang N, Wang W. Involvement of tumor necrosis factor-alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer 2010;10:419.
30. Aravena O, Pesce B, Soto L, Orrego N, Sabugo F, Wurmann P, et al. Anti-TNF therapy in patients with rheumatoid arthritis decreases Th1 and Th17 cell populations and expands IFN-γ-producing NK cell and regulatory T cell subsets. Immunobiology 2011 Dec;216(12):1256-1263.
31. Wang JY, Liu SB, Chen SY, Dobson A. Risk factors for peptic ulcer in Shanghai. Int J Epidemiol 1996 Jun;25(3):638-643.
32. Boghratian AH, Hashemi MH, Kabir A. Gender-related differences in upper gastrointestinal endoscopic findings: an assessment of 4,700 cases from Iran. J Gastrointest Cancer 2009;40(3-4):83-90.
33. Ahmed KS, Khan AA, Ahmed I, Tiwari SK, Habeeb MA, Ali SM, et al. Prevalence study to elucidate the transmission pathways of Helicobacter pylori at oral and gastroduodenal sites of a South Indian population. Singapore Med J 2006 Apr;47(4):291-296.
34. Smith A, Contreras C, Ko KH, Chow J, Dong X, Tuo B, et al. Gender-specific protection of estrogen against gastric acid-induced duodenal injury: stimulation of duodenal mucosal bicarbonate secretion. Endocrinology 2008 Sep;149(9):4554-4566.
35. Machowska A, Szlachcic A, Pawlik M, Brzozowski T, Konturek SJ, Pawlik WW. The role of female and male sex hormones in the healing process of preexisting lingual and gastric ulcerations. J Physiol Pharmacol 2004 Jul;55(Suppl 2):91-104.
36. Jaber SM. The pattern of CagA and VacA proteins in Helicobacter pylori seropositive asymptomatic children in western Saudi Arabia. Saudi Med J 2005 Sep;26(9):1372-1377.
|