Gastointestinal (GI) tract smooth muscle tumors commonly occur in the esophagus. They are uncommon in the colorectal region.1 They usually occur in the elderly with no sex or racial predilection.2,3 The exact cause and predisposing factors are still unknown; however, diet and behaviors like smoking or lack of exercise may play a role. Some literature show that colonic injury or inflammatory bowel disease may increase the risk as well.1,4 In most cases, patients are asymptomatic and these lesions are found incidentally during colonoscopy or radiological imaging.1,5 Compared to polyps, they are subepithelial lesions arising from the muscularis propria and appear mainly as sessile lesions. Occasionally, they may appear as pedunculated lesions resulting in preoperative misdiagnosis as epithelial polyps.2,5,6 Radiologically, colonic smooth muscle tumors are usually large with lobulated margins, extra-colic growth, and heterogeneous enhancement.1,7,8 Tissue biopsy is considered to be the gold standard for confirmation of the diagnosis.1 In some instances, it is difficult to differentiate between benign and malignant lesions, and therefore, a complete surgical resection of the lesion is required.9,10 This is usually curative with a good prognosis in cases of benign leiomyoma with no malignant potential or recurrence.5,6 However, in cases of leiomyosarcoma, the prognosis depends on the tumor grade and the presence of metastasis at the time of diagnosis.1,11 Although a metastatic lymphadenopathy is uncommon, a distant metastasis to the liver and abdominal cavity is well-known in cases of leiomyosarcoma.12,13 Post-surgical chemotherapy and radiotherapy are needed in such cases with 50% five-year survival.11–13 Here, we report two pathology-proven colonic leiomyomatous tumors.
Case reports
Case one
A 41-year-old woman, with no known medical issues presented to the Emergency Department, Royal Hospital, Muscat in September 2019 with a history of intermittent lower abdominal pain and pelvic fullness for the past two weeks. She was vitally stable, and an abdominal examination revealed a large pelvic non-tender hard mass. She was referred to the gynecology department where an ultrasound (US) of the pelvis was done and revealed a large complex right pelvic mass inseparable from the right ovary. Lab investigations were within normal limits and tumor markers including cancer antigen-125, carcinoembryonic antigen, α-fetoprotein, and beta-human chorionic gonadotropin were negative. Magnetic resonance imaging was done and revealed normal uterus and ovaries with a separate large mixed solid and cystic multilobular midline abdominopelvic mass in close proximity to the transverse colon which looked tethered without causing bowel obstruction. A computed tomography (CT) staging was done and showed that the corresponding mass infiltrated the transverse colon with no evidence of lymphadenopathy or distant metastasis to the lung or liver [Figure 1 a and b]. The solid component of the mass demonstrated enhancement [Figure 1 c-e].
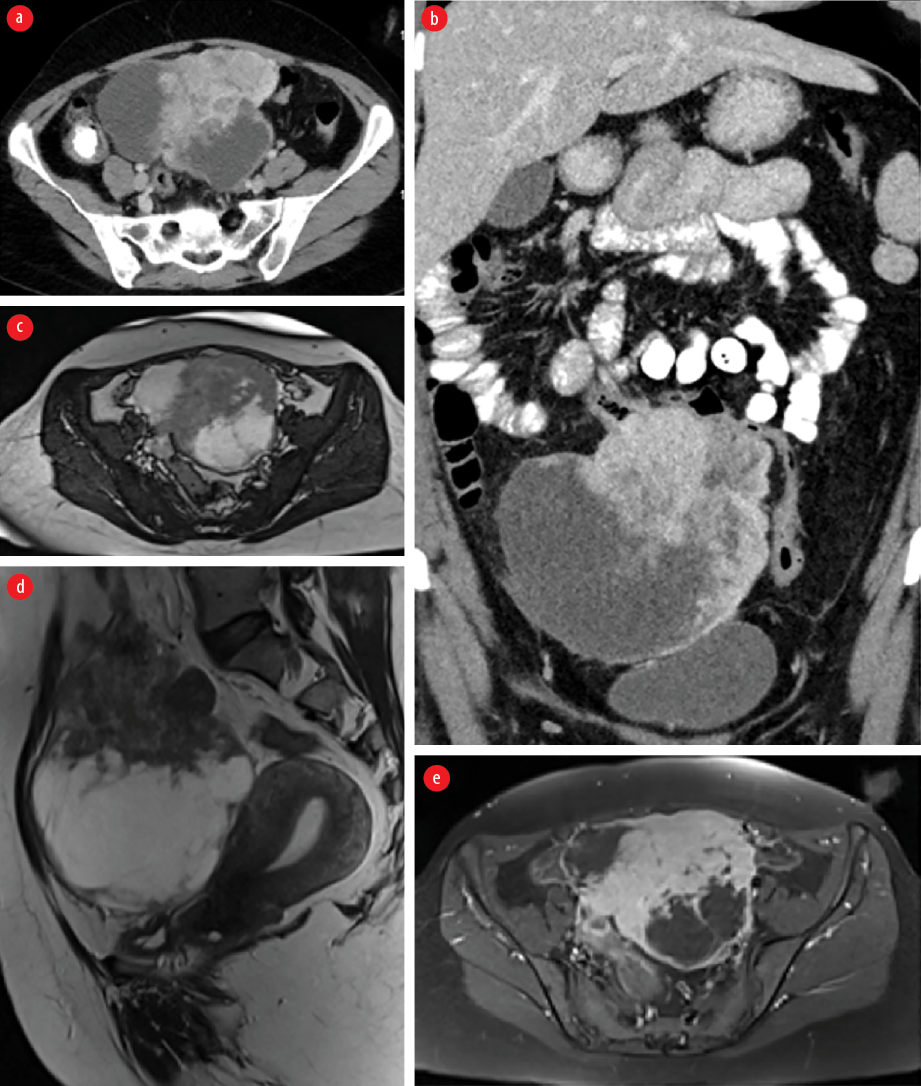 Figure 1: Contrast-enhanced CT (a) axial and (b) coronal images showing a large mixed solid and cystic multilobular midline abdominopelvic mass close to the transverse colon which is tethered without causing an obstruction. The solid component of the mass demonstrates enhancement. MRI (c) axial and (d) sagittal T2-weighted images and (e) axial T1-weighted images late gadolinium enhancement demonstrating a heterogeneous predominantly hypointense mass with cystic component and showing heterogeneous enhancement in post-contrast images. It is separated from the uterus and both ovaries.
Figure 1: Contrast-enhanced CT (a) axial and (b) coronal images showing a large mixed solid and cystic multilobular midline abdominopelvic mass close to the transverse colon which is tethered without causing an obstruction. The solid component of the mass demonstrates enhancement. MRI (c) axial and (d) sagittal T2-weighted images and (e) axial T1-weighted images late gadolinium enhancement demonstrating a heterogeneous predominantly hypointense mass with cystic component and showing heterogeneous enhancement in post-contrast images. It is separated from the uterus and both ovaries.
The patient underwent laparotomy, and excision of the mass with segmental resection of the infiltrated part of the transverse colon and primary anastomosis were done. No immediate complications were documented. Postoperatively, the patient was doing well and was discharged on postoperative day three on analgesia. The final histopathology report was a benign leiomyoma of the mesentery of transverse colon [Figure 2]. The patient was kept on a follow-up by a colorectal surgical team.
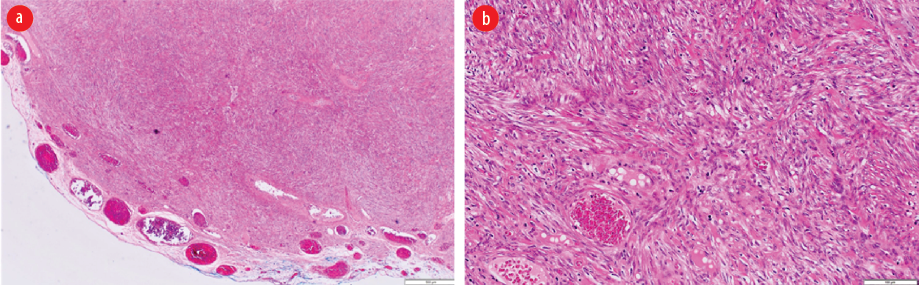 Figure 2: (a) Low power view showing a well-circumscribed spindle cell neoplasm (hematoxylin and eosin staining, magnification = 40 ×). (b) High power view showing interlacing fascicles of smooth muscle fibers lacking cytological atypia, increased mitosis, and necrosis (hematoxylin and eosin staining, magnification = 40 ×).
Figure 2: (a) Low power view showing a well-circumscribed spindle cell neoplasm (hematoxylin and eosin staining, magnification = 40 ×). (b) High power view showing interlacing fascicles of smooth muscle fibers lacking cytological atypia, increased mitosis, and necrosis (hematoxylin and eosin staining, magnification = 40 ×).
Case two
A 31-year-old man, not known to have any medical background, was presented to the surgical outpatient clinic, Royal Hospital, Muscat, in November 2015 with a history of recurrent abdominal pain for one year. He was managed conservatively with analgesia, but multiple visits were documented with the same complaints. He was vitally stable and abdominal examination revealed a large left iliac non-tender mass. Lab investigations were within normal limits. US abdomen was performed and confirmed the presence of a left iliac fossa mass. CT and MRI were done and showed aggressive looking left iliac fossa solid enhancing mass invading the left psoas muscle and peritoneum [Figure 3]. There were some tiny cystic changes within the mass. There was no evidence of distant metastasis to the lung or liver. The differential diagnoses at that time included sarcoma and lymphoma. Subsequently, US guided biopsy was performed, and the results came as smooth muscle neoplasm with atypia in which leiomyosarcoma could not be entirely excluded.
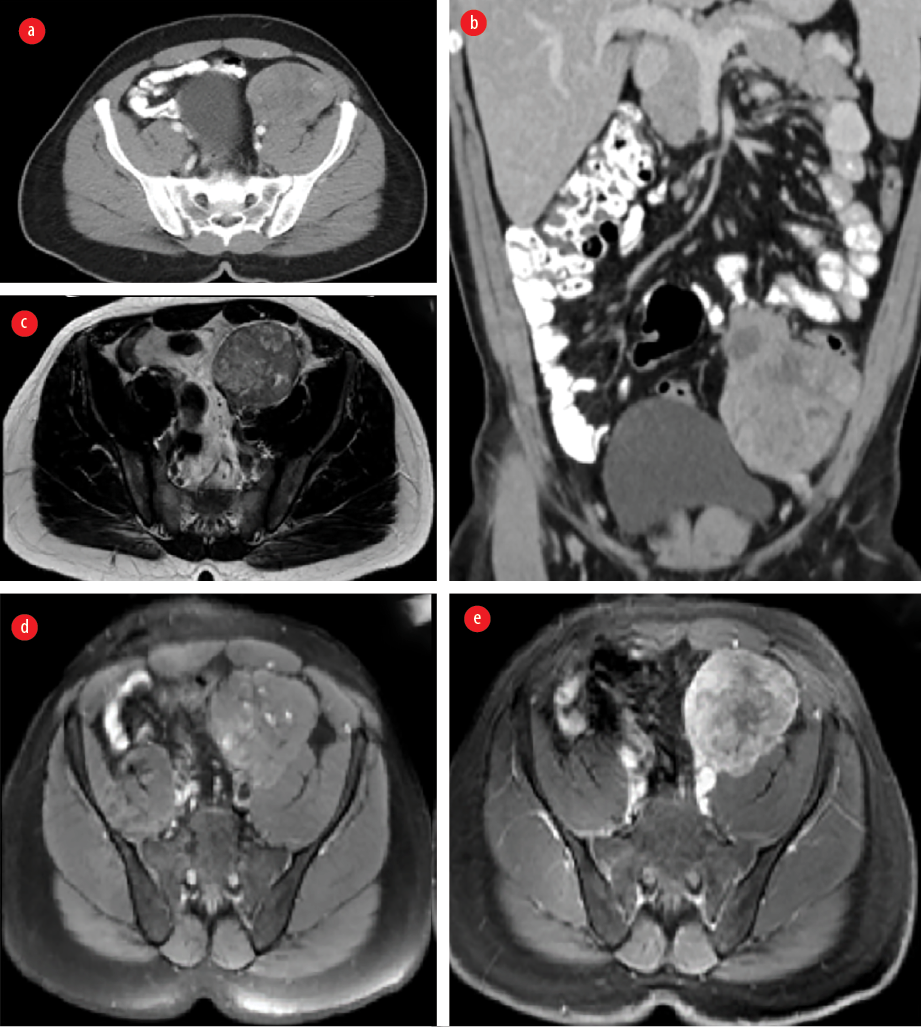 Figure 3: Contrast-enhanced CT (a) axial and (b) coronal images showing a left iliac fossa solid heterogeneous enhancing mass invading the left psoas muscle and peritoneum and is near the sigmoid colon mesentery. MRI (c) axial T2-weighted images (WI), (d) axial T1WI, (e) axial T1WI post gadolinium enhancement demonstrating a lesion of low to intermediate signal intensity with few cystic changes and showing heterogenous enhancement in post-contrast images.
Figure 3: Contrast-enhanced CT (a) axial and (b) coronal images showing a left iliac fossa solid heterogeneous enhancing mass invading the left psoas muscle and peritoneum and is near the sigmoid colon mesentery. MRI (c) axial T2-weighted images (WI), (d) axial T1WI, (e) axial T1WI post gadolinium enhancement demonstrating a lesion of low to intermediate signal intensity with few cystic changes and showing heterogenous enhancement in post-contrast images.
The patient underwent laparotomy resection of the left iliac fossa mass and segmental resection of the sigmoid colon and part of the spermatic cord. Intraoperative findings revealed a large highly vascular intraabdominal mass measured about 10 × 8 cm with an intact capsule firmly attached to the sigmoid colon. There were no immediate postoperative complications, and the patient was discharged a few days later in a stable condition. The final histopathology report was leiomyosarcoma of sigmoid colon [Figure 4]. Patient was referred to a multidisciplinary team including colorectal surgery, oncology, and radiation oncology, and multiple sessions of chemotherapy and radiotherapy were done. After two years of follow-up, he developed liver and lung metastasis as well as a metastatic mass in the right iliac fossa invading the iliacus muscle and right iliac wing. He was then started on palliative chemotherapy.
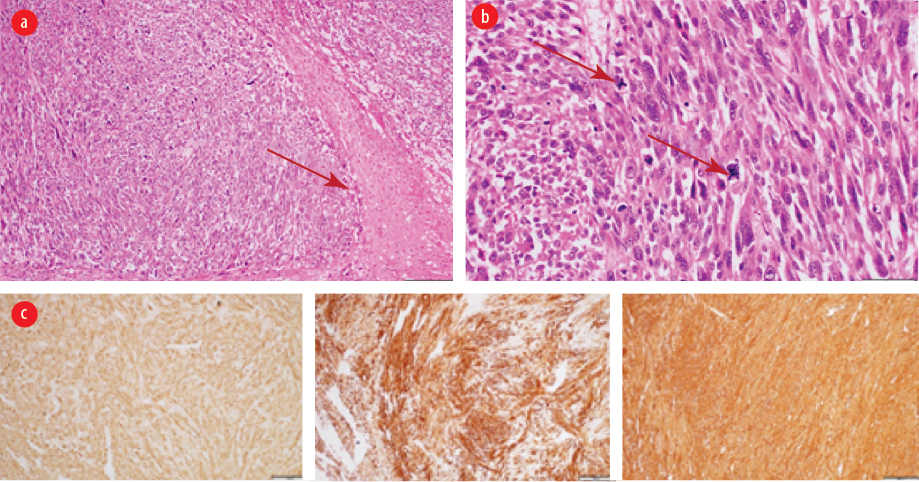 Figure 4: (a) Intermediate power view showing area of coagulative-type necrosis (arrow) (hematoxylin and eosin staining, magnification = 40 ×). (b) High power view exhibiting smooth muscle neoplasm with marked cytological atypia and scattered atypical mitosis (arrows) (hematoxylin and eosin staining, magnification = 40 ×). (c) Immunohistochemistry with the smooth muscle markers (smooth muscle actin, desmin, and H-caldesmon) are positive (hematoxylin and eosin staining, magnification = 40 ×).
Figure 4: (a) Intermediate power view showing area of coagulative-type necrosis (arrow) (hematoxylin and eosin staining, magnification = 40 ×). (b) High power view exhibiting smooth muscle neoplasm with marked cytological atypia and scattered atypical mitosis (arrows) (hematoxylin and eosin staining, magnification = 40 ×). (c) Immunohistochemistry with the smooth muscle markers (smooth muscle actin, desmin, and H-caldesmon) are positive (hematoxylin and eosin staining, magnification = 40 ×).
Discussion
Smooth muscle tumors were first described by Virchow in 1854.14 They are often seen in young women arising from the uterus. However, they can also be seen less frequently in other parts of the body including the GI tract. Esophagus and stomach are the most common locations in the GI tract smooth muscle tumors and the colon is the rarest one. Sigmoid colon, descending colon, and transverse colon are the most affected parts of the colon. Baker and Good conducted a study on GI smooth muscle tumors, which showed that 65% of leiomyomas were found in the esophagus or stomach, 23% in the small intestine, and only 3% in the colon.14,15
Mesenchymal tumors of the colon are usually asymptomatic. However, patients may present with nonspecific abdominal symptoms like pain, mass, intestinal obstruction, or hemorrhage.1,2,16 In most cases, these tumors are found incidentally during imaging or colonoscopy examination.1,4 The clinical presentation of the patient depends on the origin, size, and mass effect.1 Both of our patients presented with lower abdominal pain and a palpable mass. There are a few hypotheses that may explain this pain; 1) the pressure effect on the adjacent nerves, and 2) the contraction of smooth muscle cells. However, the exact explanation of the pain is still not clearly understood.1,3,16
Mesenchymal tumors of the digestive tract are classified by the WHO into gastrointestinal stromal tumor (GIST), lipomas, leiomyomas, leiomyosarcomas, angiosarcomas, and Kaposi sarcomas.4 The mesenchymal tumors are subepithelial tumors and represent only 1% of primary GI cancers. It is important to differentiate between the GIST and other mesenchymal tumors as they share the same features under light microscopy. This can be achieved by doing further tests using immunohistochemistry and electron microscopy.4 Colonic GISTs are rare and usually they are c-Kit (CD117) and DOG-1 positive. Mesenchymal tumors arise from smooth muscle cells and usually stain positive for smooth muscle actin (SMA) or desmin, but negative for c-Kit.1,4,17 Leiomyomas are circumscribed lesions and composed of interlacing fascicles of smooth muscle fibers. The tumor cells are bland and lack cytological atypia, frequent mitosis, and coagulative necrosis. Compared to leiomyosarcomas, which show significant cytological atypia, frequent mitosis including atypical mitosis and coagulative necrosis.4 The histology of the first patient [Figure 2] showed well-circumscribed spindle cell neoplasm with interlacing fascicles of smooth muscle fibers lacking cytological atypia, increased mitosis, and necrosis. The histology of the second patient showed smooth muscle neoplasm with marked cytological atypia and scattered atypical mitosis and area of coagulative-type necrosis [Figure 4 a and b]. Immunohistochemistry with the smooth muscle markers (SMA, desmin, and H-caldesmon) were positive [Figure 4 c-e].
The radiological findings of leiomyomatous tumors frequently overlap with other aggressive GI tumors or lymphoma. Lee et al,7 performed a study in 12 pathologically proven leiomyomatous tumors of colorectal region (two leiomyomas and 10 leiomyosarcomas) to assess their radiological features.The results revealed that most of these tumors are large in size (mean: 7.9 cm) with lobulated margins and exocolic growth, and show variable degrees of internal necrosis and heterogeneous enhancement. Dystrophic calcification sometimes can be seen.7 Another study done to evaluate the CT efficacy in differentiating between GI leiomyoma and leiomyosarcoma showed that large size (> 5 cm), lobulated contour, heterogeneous enhancement, mesenteric fat infiltration, ulceration, regional lymphadenopathy, and exophytic growth pattern are the CT features favoring malignancy with significant p-value (p < 0.005).8 In our cases, both masses are large with lobulated margins and exocolic growth, and showed heterogeneous enhancement with no evidence of lymphadenopathy or distant metastasis [Figures 1 and 3]. Therefore, these features are not very helpful in excluding malignancy, and surgical resection should be considered.
Surgical resection is the mainstay of treatment.1,9,10 Although sometimes in cases of small benign leiomyoma with no evidence of deep invasion, an endoscopic resection can be considered as an alternative to surgery given its lower costs and low rate of complications.5,6 The prognosis depends on the tumor grade and the presence of metastasis at the time of diagnosis.1,11 Although metastatic lymphadenopathy is uncommon, distant metastasis to the liver and abdominal cavity can be seen in cases of leiomyosarcoma.1,12,13
Post-surgical chemotherapy and radiotherapy were needed in some cases.13 A five-year survival is about 50% in cases of leiomyosarcoma.11,12 Leiomyoma has an excellent prognosis with no malignant potential or recurrence.2,5,6 In our patients, they underwent surgical resection of the lesions with segmental resection of the involved part of the colon. The first patient with leiomyoma did well. The second patient received multiple sessions of radiotherapy but after two years of follow-up, he developed metastasis to liver, lung, and right iliac fossa, which necessitated palliative chemotherapy.
Conclusion
Colonic leiomyomatous tumors are rare and usually discovered incidentally during radiological imaging or colonoscopy. Radiological findings can be non-specific but the presence of certain features including the large size and extra-colic growth favors the diagnosis. CT can help to differentiate between benign leiomyoma and leiomyosarcoma; however, tissue diagnosis is the confirmatory method. Surgical resection is the mainstay of treatment with or without radiotherapy and chemotherapy in some cases of leiomyosarcoma.
Disclosure
The authors declared no conflicts of interest. Informed consent was obtained from the patient.
references
- 1. Alpert L, Al-Sabti R, Graham RP, Pai RK, Gonzalez RS, Zhang X, et al. Smooth muscle tumors of the gastrointestinal tract: an analysis of prognostic features in 407 cases. Mod Pathol 2020 Jul;33(7):1410-1419.
- 2. Ikeda A, Iwamuro M, Tanaka T, Inokuchi T, Nakarai A, Sugihara Y, et al. Two cases of leiomyoma in the colon masquerading as other types of colonic pedunculated polyps. Case Rep Gastrointest Med 2018;2018:8272313.
- 3. Lugo-Fagundo E, Fishman EK. Colorectal leiomyosarcoma: a case report. Radiol Case Rep 2022;17(8):2812-2814.
- 4. Miettinen M, Sarlomo-Rikala M, Sobin LH. Mesenchymal tumors of muscularis mucosae of colon and rectum are benign leiomyomas that should be separated from gastrointestinal stromal tumors–a clinicopathologic and immunohistochemical study of eighty-eight cases. Mod Pathol 2001 Oct;14(10):950-956.
- 5. Lee SH, Huh GY, Cheong YS. A case of endoscopic resection of a colonic semipedunculated leiomyoma. J Korean Soc Coloproctol 2011 Aug;27(4):215-219.
- 6. Chow WH, Kwan WK, Ng WF. Endoscopic removal of leiomyoma of the colon. Hong Kong Med J 1997;3:325-327.
- 7. Lee SH, Ha HK, Byun JY, Kim AY, Cho KS, Lee YR, et al. Radiological features of leiomyomatous tumors of the colon and rectum. J Comput Assist Tomogr 2000;24(3):407-412.
- 8. Chun HJ, Byun JY, Chun KA, Rha SE, Jung SE, Lee JM, et al. Gastrointestinal leiomyoma and leiomyosarcoma: CT differentiation. J Comput Assist Tomogr 1998;22(1):69-74.
- 9. Bananzadeh A, Mokhtari M, Sohooli M, Shekouhi R. Two cases of primary leiomyosarcoma of sigmoid colon treated with laparoscopic surgery: a case report and a review of literature. Int J Surg Case Rep 2021 Oct;87:106420.
- 10. Yahagi M, Ishii Y, Hara A, Watanabe M. Laparoscopic surgery to treat leiomyosarcomas of the sigmoid colon: a case report and literature review. Surg Case Rep 2019;5(1):20.
- 11. Devriendt S, Leman G, Vanrykel F. Primary leiomyosarcoma of the colon: a case report and review of the literature. Acta Chir Belg 2020 Oct;120(5):353-356.
- 12. Yang J. Primary leiomyosarcoma in the colon: a case report. Medicine (Baltimore) 2018 Feb;97(7):e9923.
- 13. Akutsu D, Mizokami Y, Suzuki H, Terasaki M, Narasaka T, Kaneko T, et al. A rare case of colonic leiomyosarcoma in association with ulcerative colitis. Intern Med 2016;55(19):2799-2803.
- Kandhasamy CS, Sangwan A, Sahoo AK, Gunasekaran G, Sahani N, Raju TR, et al. An uncommon presentation of leiomyoma cecum as a subcutaneous abscess of the right flank. Cureus. 2018;10(10):e3432.
- Baker HL, Jr, Good CA. Smoothmuscle tumors of the alimentary tract; their roentgen manifestations. Am J Roentgenol Radium Ther Nucl Med. 1955;74:246–255.
- 14. Sagnotta A, Sparagna A, Uccini S, Mercantini P. Giant extraluminal leiomyoma of the colon: rare cause of symptomatic pelvic mass. Int Surg 2015 May;100(5):805-808.
- 15. Crystal JS, Korderas K, Schwartzberg D, Tizio SC, Zheng M, Parker G. Primary leiomyosarcoma of the colon: a report of two cases, review of the literature, and association with immunosuppression for IBD and rheumatoid arthritis. Case Rep Surg 2018;2018:6824643.