In March 2020, the World Health Organization (WHO) declared the SARS-CoV-2 virus as a pandemic infection, leading to COVID-19.1–3 As of 5 June 2023, over 767 million people worldwide have been infected, with more than 6.9 million deaths reported.1 Oman reported a total of 399 449 COVID-19 cases and over 4600 confirmed deaths.1 The clinical features of the disease are well-established, including fever, headache, body ache, cough, shortness of breath, loss of smell and taste, and gastrointestinal symptoms such as diarrhea and vomiting.4 Additionally, various complications have been reported, such as respiratory failure, cardiac issues, renal problems, neurological manifestations, thrombotic events, and hepatic complications.5–8 Post-COVID-19 syndrome, also known as long COVID, typically refers to persistent symptoms that last beyond four weeks of disease onset.9–11 Recently, WHO defined long COVID as persistent symptoms beyond three months from the onset of COVID-19 that persist for two months and cannot be explained by another diagnosis in patients who had probable or confirmed COVID-19.12 The epidemiology of long COVID has been described in many studies from various parts of the world with variable results due to variations in methodology, patients included, and follow-up period.9,10 The incidence of post-COVID-19 symptoms in American and European studies ranges from 30–90% at six months.13–15 Although common residual symptoms including fatigue (40%), shortness of breath (36%), atypical chest pain (13.1%), a persistent cough (16.9%), and anosmia (11%) have been described in several studies,16–18 there are no studies on the prevalence of post-COVID-19 symptoms in Oman. This study aimed to assess the incidence of COVID-19-related symptoms in patients with mild, severe, and critical disease in Oman.
Methods
This bidirectional observational cohort study included adult patients aged ≥ 18 years admitted to the Armed Forces Hospital with SARS-CoV-2 infection confirmed by reverse transcription polymerase chain reaction between July 2020 and June 2022. Patients were admitted to the COVID-19 ward if they had bilateral lung infiltrates and oxygen desaturation below 93% or required oxygen for other indications related to severe COVID-19. Patients were admitted to the intensive care unit (ICU) or transferred from the COVID-19 ward to ICU if they had critical disease based on WHO guidelines were also included.19 Healthcare workers (HCWs) with mild disease were included as a comparative group. HCWs were requested to complete an online questionnaire, and the admitted patients were asked to attend post-COVID-19 follow-up clinics at weeks six and 12 post-discharge, during which patients answered questionnaires that included demographic data, past medical history, presenting symptoms that led to hospital admission, and residual symptoms. Chest X-rays and abnormal blood investigations were repeated as clinically indicated. Other investigations, such as electrocardiogram, echocardiography, and chest computed tomography, were performed according to clinical indications.
Patients who died, those who were aphasic or had poor mental status, patients who were bedridden and could not be followed-up at an outpatient clinic, and those who did not attend the follow-up appointment were excluded from the study.
A scoring system modified from Galal et al,20 was created based on 18 symptoms during the acute and post-COVID-19 stages. A scale of 0–1 was used for each symptom (0 = absent and 1 = present). The range of the overall score was 0–18, with a high score indicating more severe symptoms.
Statistical analyses were performed using STATA software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.). Categorical data were presented as numbers and percentages, while continuous data were reported as means±SD and/or median (interquartile range) and tested for normality using the Shapiro-Wilk test. Continuous variables were analyzed using either Students’ t-test or Mann-Whitney U test for comparison of two groups whenever appropriate. Chi-squared test was used for the comparison of binary variables. Spearman’s correlation was used to find the correlation between symptom scores in acute and post-COVID-19 stages. Univariate and multivariate-adjusted logistic regression analyses were performed to identify baseline determinants of persistent post-COVID-19 symptoms. In all statistical tests, a p-value < 0.05 was considered statistically significant.
The study was approved by the ethical committee of Armed Forces Hospital, Oman (IRB number: FMS-MREC 027/2020). Verbal informed consent was taken from all patients at the clinic.
Results
Our study included 468 patients diagnosed with COVID-19 and followed-up at the post-COVID-19 clinic. The mean age of the enrolled participants was 48.4 years, with 209 (44.7%) males and 259 (55.3%) females [Table 1].
Table 1: Baseline demographics and clinical data of the study participants, categorized into three groups based on the severity of COVID-19 disease (N = 468).
|
Age, years
|
41.9 ± 18.8
|
54.6 ± 14.1
|
49.3 ± 11.8
|
|
Male gender
|
35 (16.9)
|
140 (67.3)
|
34 (64.1)
|
|
BMI
|
25.9 ± 4.2
|
30.5 ± 6.6
|
30.5 ± 4.8
|
|
Vaccination status,
|
No available data
|
10 (4.8)
|
5 (9.4)
|
|
Number of doses
|
|
One dose
|
No available data
|
6 (2.9)
|
4 (7.5)
|
|
Two doses
|
|
4 (1.9)
|
1 (1.9)
|
|
Oxygen requirements
|
|
None
|
None
|
22 (10.6)
|
0 (0.0)
|
|
2–5
|
|
118 (56.3)
|
6 (11.3)
|
|
5–10
|
|
40 (19.2)
|
4 (7.5)
|
|
15
|
|
26 (12.5)
|
43 (81.1)
|
|
Persistent COVID-19
|
55 (26.6)
|
102 (49.0)
|
29 (54.7)
|
|
Medical background
|
|
Diabetes mellitus,
|
11 (5.3)
|
95 (45.7)
|
16 (30.2)
|
|
Hypertension,
|
9 (4.3)
|
99 (47.6)
|
23 (43.4)
|
|
Heart disease
|
1 (0.5)
|
26 (12.5)
|
6 (11.3)
|
|
Dyslipidemia,
|
0 (0.0)
|
80 (38.5)
|
18 (34.0)
|
|
Chronic lung disease
|
1 (0.5)
|
13 (6.3)
|
6 (11.3)
|
|
Smoking
|
1 (0.5)
|
0 (0.0)
|
0 (0.0)
|
|
Renal failure
|
2 (1.0)
|
5 (2.4)
|
4 (7.5)
|
|
Liver disease
|
0 (0.0)
|
2 (1.0)
|
0 (0.0)
|
|
CVA
|
0 (0.0)
|
2 (1.0)
|
1 (1.9)
|
Numeric values are presented as means ± SD, and categorical values are expressed as frequencies (percentages).
HCW: healthcare workers; ICU: intensive care unit; BMI: body mass index; CVA: cerebrovascular accident.
A majority of patients, 445 (95.1%), did not receive COVID-19 vaccination. Among the hospitalized patients, 131 (28.0%) patients had hypertension, 122 (26.1%) had diabetes, and 98 (20.9%) had dyslipidemia. Among all included patients, 207 (44.2%) experienced mild disease that did not require hospitalization, while 261 (55.8%) were admitted. Among the admitted patients, 53 (11.3%) required ICU admission.
The most common presenting symptoms of acute COVID-19 for both admitted and HCWs during the acute phase were fever (385; 8.3%), fatigue (336; 71.8%), and cough (303; 64.7%). The mean duration from symptom onset to the follow-up visit was 68.0 days. The mean total score of acute stage symptoms was 8.0±3.5, whereas the post-COVID-19 symptom score was 1 (p < 0.001), as shown in Table 2.
Table 2: Comparison of baseline symptoms according to patients’ groups (N = 468).
|
Fever, 385/8
|
156 (75.4)
|
2 (1.0)
|
180 (86.5)
|
5 (2.4)
|
49 (92.5)
|
1 (1.9)
|
|
Cough, 303/68
|
112 (54.1)
|
14 (6.8)
|
149 (71.6)
|
39 (18.8)
|
42 (79.2)
|
15 (28.3)
|
|
Sputum, 167/44
|
48 (23.2)
|
7 (3.4)
|
92 (44.2)
|
25 (12.0)
|
27 (50.9)
|
12 (22.6)
|
|
Shortness of breath, 271/75
|
48 (23.2)
|
7 (3.4)
|
179 (86.1)
|
48 (23.1)
|
44 (83.0)
|
20 (37.7)
|
|
Chest pain, 200/49
|
42 (20.3)
|
7 (3.4)
|
127 (61.1)
|
31 (14.9)
|
31 (58.5)
|
11 (20.8)
|
|
Loss of smell, 230/22
|
131 (63.3)
|
12 (5.8)
|
78 (37.5)
|
8(3.8)
|
21 (39.6)
|
2 (3.8)
|
|
Loss of taste, 233/17
|
126 (60.9)
|
9 (4.3)
|
85 (40.9)
|
5 (2.4)
|
22 (41.5)
|
3 (5.7)
|
|
Rhinitis, 134/14
|
79 (38.2)
|
3 (1.4)
|
45 (21.6)
|
10 (4.8)
|
10 (18.9)
|
1 (1.9)
|
|
Red eye, 53/7
|
22 (10.6)
|
0 (0.0)
|
27 (13.0)
|
7 (3.4)
|
4 (7.5)
|
0 (0.0)
|
|
Diarrhea, 116/8
|
52 (25.1)
|
1 (0.5)
|
48 (23.1)
|
6 (2.9)
|
16 (30.19)
|
1 (1.9)
|
|
Nausea, 121/1
|
46 (22.2)
|
3 (1.4)
|
62 (29.8)
|
10 (4.8)
|
13 (24.5)
|
0 (0.0)
|
|
Vomiting, 68/4
|
28 (13.5)
|
0 (0.0)
|
33 (15.9)
|
4 (1.9)
|
7 (13.2)
|
0 (0.0)
|
|
Fatigue, 336/54
|
148 (71.5)
|
13 (6.3)
|
150 (72.1)
|
29 (13.9)
|
38 (71.7)
|
12 (22.6)
|
|
Vertigo, 150/26
|
57 (27.5)
|
11 (5.3)
|
75 (36.1)
|
12 (5.8)
|
18 (334.0)
|
3 (5.7)
|
|
Headache, 264/33
|
148 (71.5)
|
12 (5.8)
|
91 (43.8)
|
18 (8.7)
|
25 (47.0)
|
3 (5.7)
|
|
Weakness, 53/17
|
1 (0.5)
|
0 (0.0)
|
45 (21.6)
|
10 (4.8)
|
7 (13.2)
|
7 (13.2)
|
|
Joints pain, 271/60
|
128 (61.8)
|
9 (4.3)
|
113 (54.3)
|
36 (17.3)
|
30 (56.6)
|
15 (28.3)
|
HCW: healthcare workers; ICU: intensive care unit.
At the follow-up time point, 60.3% of the patients (n = 282) had a symptom score of 0, indicating the absence of persistent symptoms. On the other hand, 39.7% of the patients (n = 186) had a symptom score of at least 1, indicating the presence of persistent symptoms. The most commonly reported persistent symptoms during the follow-up visit were shortness of breath reported by 75 (16.0%) patients, followed by cough (n = 68; 14.5%), and joints pain (n = 60; 12.8%). A strong positive correlation was observed between the symptom score during the acute attack and post-COVID-19 stage (p < 0.001, r = 0.20) as illustrated in Figure 1. There was a significant reduction in baseline symptoms scores at the follow-up time point across different patient subgroups including age, gender, body mass index, and severity of baseline illness, as shown in Table 3. The most frequent pre-existing comorbidities associated with persistent post-COVID-19 symptoms and symptom scores among the study population were hypertension (p = 0.003) followed by chronic pulmonary disorders (p = 0.004), dyslipidemia (p = 0.010) and cardiac diseases that include ischemic heart disease, atrial fibrillation, and heart failure
(p = 0.040) as shown in Table 4.
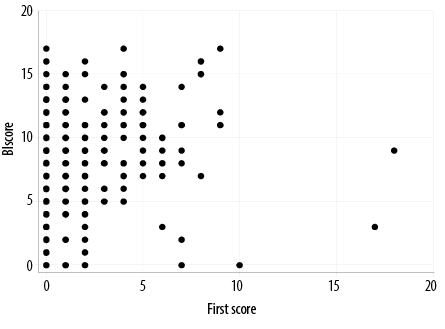 Figure 1: Correlation between symptom scores during acute and post-COVID-19 stages.
Figure 1: Correlation between symptom scores during acute and post-COVID-19 stages.
Table 3: Comparison of baseline symptom scores among different patient groups at follow-up (N = 468).
|
Age, years
|
|
< 25
|
16 (3.4)
|
8.1 ± 3.2
|
0 (0, 3.5)
|
< 0.001*
|
|
25–50
|
285 (60.9)
|
7.9 ± 3.6
|
0 (0, 1)
|
|
> 50
|
172 (36.8)
|
7.5 ± 3.5
|
0 (0, 2)
|
|
Gender
|
|
Male
|
259 (55.3)
|
7.9 ± 3.7
|
0 (0, 1)
|
< 0.001 *
|
|
Female
|
209 (44.7)
|
7.5 ± 3.3
|
0 (0, 2)
|
|
BMI^
|
|
Underweight
|
6 (1.3)
|
9.6 ± 3.9
|
0 (0, 2)
|
0.030
|
|
Normal
|
105 (22.4)
|
7.3 ± 3.5
|
0 (0, 1)
|
< 0.001
|
|
Overweight
|
184 (39.3)
|
7.9 ± 3.7
|
0 (0, 1.5)
|
< 0.001
|
|
Obese
|
113 (24.1)
|
7.0 ± 3.1
|
0 (0, 2)
|
< 0.001*
|
|
Severity of illness
|
|
|
|
|
|
Mild (HCW)
|
207 (22.2)
|
7.3 ± 3.6
|
0 (0, 1)
|
< 0.001
|
|
Moderate (non-ICU)
|
208 (44.4)
|
8.1 ± 3.5
|
0 (0, 2)
|
|
Severe (ICU)
|
53 (11.3)
|
8.1 ± 3.3
|
2 (0, 4)
|
|
Baseline symptoms score
|
|
|
|
|
|
< 5 (n = 128)
|
128 (27.4)
|
3.3 ± 1.4
|
0 (0, 1)
|
|
5–10 (n = 231)
|
231 (49.4)
|
6.9 ± 1.3
|
0 (0, 2)
|
*Compare baseline to first follow-up score.
^Missing for 60 patients.
Numeric values are presented as means±SD, and categorical values are expressed as frequencies (percentages). The p-values indicate the significance of the changes in symptom scores from baseline to follow-up within each patient group.
BMI: body mass index; HCW: healthcare workers; ICU: intensive care unit.
Table 4: Median post-COVID-19 symptom score in patients according to comorbidities (N = 468).
|
Diabetes mellitus
|
0 (0, 1); n = 346
|
0 (0, 2); n = 122
|
0.180
|
|
Hypertension
|
0 (0, 1); n = 337
|
1 (0, 2); n = 131
|
0.003
|
|
Cardiac (IHD, CCF, AF)
|
0 (0, 2); n = 436
|
1 (0, 3.5); n = 32
|
0.040
|
|
DLP
|
0 (0, 1); n = 370
|
0 (0, 2); n = 98
|
0.010
|
|
Chest disease
|
0 (0, 2); n = 448
|
2 (0, 5); n = 20
|
0.004
|
|
Renal failure
|
0 (0, 2); n = 457
|
0 (0, 0); n = 11
|
0.140
|
|
Liver disease
|
0 (0, 2); n = 466
|
1.5 (0, 3); n = 2
|
0.680
|
|
CVA
|
0 (0, 2); n = 465
|
0 (0, 0); n = 3
|
0.180
|
|
Thyroid disease
|
0 (0, 3); n = 460
|
0.5 (0, 1.5); n = 8
|
0.690
|
IHD: ischemic heart disease; CCF: congestive cardiac failure; AF: atrial fibrillation; DLP: dyslipidemia; CVA: cerebrovascular accident; IS: immunosuppressant.
Univariate logistic regression analyses revealed that age > 50 years (OR = 1.51, 95% CI: 1.03–2.20; p = 0.037), higher baseline symptom score (OR = 1.10, 95% CI: 1.04–1.16; p < 0.001), severe disease (OR = 2.66, 95% CI: 1.76–4.01; p < 0.001), and critical disease (OR = 3.33, 95% CI: 1.79–6.22; p < 0.001) were significantly associated with persistent COVID-19 symptoms. Additionally, hypertension (OR = 1.8, 95% CI: 1.22–2.76; p = 0.004) showed a significant association with persistent symptoms. The multivariate-adjusted logistic regression model showed that higher baseline symptom score (OR = 1.10, 95% CI: 1.04–1.17; p = 0.001) and hypertension (OR = 1.71, 95% CI: 1.00–2.91; p = 0.050) were independent determinants for persistent COVID-19 symptoms, as demonstrated in Table 5.
Table 5: Logistic regression model for predictors of persistent COVID-19 (N = 468).
|
Age > 50 years
|
1.51 (1.03–2.20)
|
0.037
|
1.21 (0.75–1.95)
|
0.440
|
|
Male gender
|
1.38 (0.95–2.00)
|
0.090
|
1.37 (0.93–2.04)
|
0.110
|
|
Baseline symptom score
|
1.10 (1.04–1.16)
|
< 0.001
|
1.10 (1.04–1.17)
|
0.001
|
|
Disease severity at baseline
|
|
Mild
|
Ref
|
Ref
|
NA
|
NA
|
|
Moderate
|
2.66 (1.76–4.01)
|
< 0.001
|
|
|
|
Severe
|
3.33 (1.79–6.22)
|
< 0.001
|
|
|
|
Diabetes mellitus
|
1.29 (0.84–1.96)
|
0.240
|
0.81 (0.47–1.38)
|
0.430
|
|
Hypertension
|
1.8 (1.22–2.76)
|
0.004
|
1.71 (1.00–2.91)
|
0.050
|
|
Chest disease
|
2.36 (0.95–5.89)
|
0.065
|
1.52 (0.58–4.03)
|
0.400
|
OR: odds ratio.
Univariate logistic regression revealed that female gender (OR = 1.56, 95% CI: 1.12–1.31; p = 0.040) and higher baseline symptoms score (OR = 1.21, 95% CI: 1.02–1.31; p < 0.001) were positively associated with persistent COVID-19. Multivariate-adjusted logistic regression model showed that a higher baseline symptoms score (OR = 1.20, 95% CI: 1.09–1.31; p < 0.001), absence of diabetes (OR = 2.14, 95% CI: 1.05–4.33; p = 0.040) were independent determinants for persistent COVID-19 [Table 6].
Table 6: Logistic regression model for predictors of persistent COVID-19 among admitted patients
(n = 261).
|
Age > 50
|
0.98 (0.60–1.60)
|
0.940
|
1.54 (0.77–3.06)
|
0.220
|
|
Male gender
|
0.52 (0.31–0.88)
|
0.02
|
0.67 (0.34–1.33)
|
0.250
|
|
Baseline symptom score
|
1.21 (1.12–1.31)
|
< 0.001
|
1.20 (1.09–1.31)
|
< 0.001
|
|
Severe disease at baseline
|
1.26 (0.69–2.30)
|
0.460
|
NA
|
NA
|
|
Diabetes mellitus
|
0.66 (0.40–1.07)
|
0.090
|
0.47 (0.23–0.95)
|
0.040
|
|
Hypertension
|
1.11 (0.69–1.81)
|
0.660
|
1.42 (0.70–2.87)
|
0.330
|
|
Chest disease
|
1.77 (0.67–4.65)
|
0.250
|
0.67 (0.20–2.32)
|
0.530
|
|
Cardiac disease
|
1.14 (0.54–2.40)
|
0.720
|
1.21 (0.44–3.30)
|
0.710
|
|
CRP
|
0.99 (0.99–1.00)
|
0.190
|
0.99 (0.99–1.00)
|
0.320
|
|
Ferritin
|
0.99 (0.99–1.00)
|
0.900
|
0.99 (0.99–1.00)
|
0.680
|
|
D-dimer
|
1.00 (0.99–1.01)
|
0.470
|
1.00 (0.99–1.00)
|
0.970
|
|
LDH
|
0.99 (0.99–1.00)
|
0.560
|
1.00 (0.98–1.04)
|
0.510
|
CRP: C-reactive protein; LDH: lactate dehydrogenase.
Among the hospitalized patients, 42 developed COVID-19-related complications. The most encountered post-COVID complications were pulmonary embolism (7.7%), denovo diabetes mellitus (1.9%), and lung fibrosis (8.0%).
Discussion
The definition of long COVID has evolved over the course of the COVID-19 pandemic.21 Initially, studies observed symptoms persisting beyond four weeks in 13.3% of patients,10 but later investigations explored symptoms persisting beyond one year.17 This study presents the first detailed examination of the long-term consequences of COVID-19 infection in a large group of Omani patients, including those who were hospitalized with severe and critical disease and HCWs with mild disease. Our study showed that 39.7% of patients experienced at least one residual symptom, with breathlessness, cough, and joint pain being the most common at two months from the onset of symptoms. A study from Saudi Arabia showed that the incidence of post-COVID-19 syndrome was 45% which is comparable to our findings.22 Another study from Saudi Arabia demonstrated a lower incidence of 22%.23 Patients who had severe and critical disease had more residual symptoms, aligning with other international studies.16,24,25 Fatigue was a common residual symptom in many studies and ranged from 13% to 77% depending on study inclusions and follow-up period.26,27 This was not the case in our study as fatigue was a common presenting symptom during the acute illness (71.8%) but not in the post-COVID period. The same observations have been noted in the studies from Saudi Arabia, with fatigue being reported in 11.5% in one study and 19% in the other.22,23 This could be explained by the cultural background of our patients who always try to underreport non-physical symptoms such as fatigue.
The most prevalent comorbidities in the study cohort were diabetes, hypertension, and dyslipidemia, consistent with observations from other studies in the region.22,23,28 Post-COVID-19 syndrome has been related to older age, diabetes, hypertension, dyslipidemia, and cardiovascular disease which is similar to our findings.29 Post-COVID-19 symptoms were also seen in HCWs with mild disease, impacting their work performance and return to normal duties.30 Although previous studies have shown that HCWs had more residual symptoms compared to non-healthcare patients,29 however, this was not apparent in our study, probably due to the fact that HCWs are of younger age at our institution and very few had comorbidities.
Moreover, 21 patients developed post-COVID-19 pulmonary fibrosis (PCPF), primarily in severe and critical disease, and those who spent extended days in hospital with an average of 37 days. Similar risk factors for PCPF have been reported in many other studies.31,32 In a recent meta-analysis study, the authors have shown that the prevalence of PCPF was 44.9% for COVID-19 survivors.33 They reported that chronic obstructive pulmonary disease was the only comorbidity associated with PCPF. Although some laboratory blood results (pre-discharge) were abnormal in many patients, however, most had normal results during follow-up visits, which is in line with prior research.34,35
The number of newly diagnosed diabetes post-COVID-19 in our cohort was small (1.9%) compared to 14%, reported by a large meta-analysis of eight studies that investigated newly diagnosed diabetes in admitted COVID-19 patients.36
Furthermore, our study included patients with mild, severe, and critical disease, which covered various severity grades of COVID-19 infection. In addition, one of the strengths of our study is that our patients were interviewed and clinically evaluated, unlike other studies that mainly relied on administering questionnaires to patients over the phone or conveyed through social media tools.37
The limitations of our study include the relatively small number of patients attending follow-up clinics despite multiple reminders and calls. In addition, we also did not carry out lung function tests in patients who reported persistent respiratory symptoms because the lung function lab was not functioning during the study period due to COVID-19 restrictions. Moreover, there might be a recall bias in the HCWs group with mild disease. Also, there was no systematic assessment of the breathlessness severity using a known index. We also did not include sleep-related and mental health-related sequelae.
Conclusion
This is the first comprehensive study of post-COVID-19 syndrome in the Omani population. The study sheds light on the significant percentage of residual symptoms in patients two months after discharge and underscores the need for systematic follow-up and management of individuals recovering from acute COVID-19. Predictors of post-COVID-19 syndrome include female gender, older age, severe disease, and hypertension. HCWs who have significant residual symptoms also require support to help them go back to work and to identify those who require modification of their work environment to be able to cope with their work challenges.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We thank all the patients who participated in this study, the study nurses, and the staff who helped us to conduct this study.
references
- 1. Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity (Silver Spring) 2010 Aug;18(8):1510-1515.
- 2. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020 Mar;382(13):1199-1207.
- 3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020 Apr;382(18):1708-1720.
- 4. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al; Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020 May;323(20):2052-2059.
- 5. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020 Mar;323(11):1061-1069.
- 6. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA 2020 Apr;323(16):1612-1614.
- 7. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020 Apr;382(17):e38.
- 8. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020 Jun;77(6):683-690.
- 9. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 2021 Oct;92:55-70.
- 10. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med 2021 Apr;27(4):626-631.
- 11. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021 Aug;11(1):16144.
- 12. World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. 2021 [cited 2023 June 30]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- 13. Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J 2021 Aug;58(2):2101763.
- 14. Heightman M, Prashar J, Hillman TE, Marks M, Livingston R, Ridsdale HA, et al. Post-COVID-19 assessment in a specialist clinical service: a 12-month, single-centre, prospective study in 1325 individuals. BMJ Open Respir Res 2021 Nov;8(1):e001041.
- 15. Shoucri SM, Purpura L, DeLaurentis C, Adan MA, Theodore DA, Irace AL, et al. Characterising the long-term clinical outcomes of 1190 hospitalised patients with COVID-19 in New York City: a retrospective case series. BMJ Open 2021 Jun;11(6):e049488.
- 16. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021 May;4(5):e2111417.
- 17. Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis 2022 Apr;74(7):1191-1198.
- 18. Al-Jahdhami I, Al-naamani K, Al-Mawali A, Bennji SM. Respiratory complications after COVID-19. Oman Med J 2022 Jan;37(1):e343.
- 19. World Health Organization. Therapeutics and COVID-19: living guideline. 10th ed; 2022.
- 20. Galal I, Hussein AA, Amin MT, Saad MM, Zayan HE, Abdelsayed MZ, et al. Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score. Egypt J Bronchol 2021;15(1):10.
- 21. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 2021 Apr;27(4):601-615.
- 22. Alghamdi SA, Alfares MA, Alsulami RA, Alghamdi AF, Almalawi AM, Alghamdi MS, et al. Post-COVID-19 syndrome: incidence, risk factor, and the most common persisting symptoms. Cureus 2022 Nov;14(11):e32058.
- 23. AlRadini FA, Alamri F, Aljahany MS, Almuzaini Y, Alsofayan Y, Khan A, et al. Post-acute COVID-19 condition in Saudi Arabia: a national representative study. J Infect Public Health 2022 May;15(5):526-532.
- 24. Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Sechenov StopCOVID Research Team. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy 2021 Sep;51(9):1107-1120.
- 25. Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect 2021 Oct;27(10):1507-1513.
- 26. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021 Feb;4(2):e210830.
- 27. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021 Aug;38:101019.
- 28. Tleyjeh IM, Saddik B, AlSwaidan N, AlAnazi A, Ramakrishnan RK, Alhazmi D, et al. Prevalence and predictors of post-acute COVID-19 syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One 2021;16(12):e0260568.
- 29. Senjam S, Balhara YP, Kumar P, Nichal N, Manna S, Madan K, et al. Assessment of post COVID-19 health problems and its determinants in North India: a descriptive cross-sectional study. SSRN Electron J 2021.
- 30. Praschan N, Josephy-Hernandez S, Kim DD, Kritzer MD, Mukerji S, Newhouse A, et al. Implications of COVID-19 sequelae for health-care personnel. Lancet Respir Med 2021 Mar;9(3):230-231.
- 31. Aul DR, Gates DJ, Draper DA, Dunleavy DA, Ruickbie DS, Meredith DH, et al. Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir Med 2021 Nov;188:106602.
- 32. Ali RM, Ghonimy MB. Post-COVID-19 pneumonia lung fibrosis: a worrisome sequelae in surviving patients. Egypt J Radiol Nucl Med 2021;52(1):101.
- 33. Hama Amin BJ, Kakamad FH, Ahmed GS, Ahmed SF, Abdulla BA, Mohammed SH, et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond) 2022 May;77:103590.
- 34. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al; ARC Study Group. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021 Apr;76(4):396-398.
- 35. Varghese J, Sandmann S, Ochs K, Schrempf IM, Frömmel C, Dugas M, et al. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep 2021 Jun;11(1):12775.
- 36. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab 2021 Mar;23(3):870-874.
- 37. Garout MA, Saleh SA, Adly HM, Abdulkhaliq AA, Khafagy AA, Abdeltawab MR, et al. Post-COVID-19 syndrome: assessment of short- and long-term post-recovery symptoms in recovered cases in Saudi Arabia. Infection 2022 Dec;50(6):1431-1439.