Herpes simplex virus (HSV) encephalitis is the most prevalent infectious cause of sporadic encephalitis,1 with an estimated frequency of 2–4 occurrences per 1 000 000 people worldwide. The morbidity and mortality rates are high with HSV encephalitis even after anti-viral therapy with acyclovir.2 Although the disease frequently has a monophasic course, 5–27% of individuals experience a neurologic recurrence within a few weeks or months. When symptoms of post-HSV encephalitis recur, they may be a real relapse (cerebrospinal fluid (CSF) polymerase chain reaction (PCR) positive for HSV) or an immune-mediated illness (CSF negative for HSV).3 The immune-mediated presentation of HSV encephalitis is frequently associated with N-methyl-D-aspartate receptor (NMDAR) antibodies. It manifests as a multistage sickness, with most patients first experiencing a prodromal illness that resembles a viral infection, then psychiatric symptoms such as altered irritability, behavior, speech, psychosis, or emotional lability.4 Neurologic symptoms (seizures, catatonia, and mutism), followed by autonomic instability and dyskinesia, might develop as the illness progresses.5 The study reports two cases of post-herpes encephalitis to highlight the importance of early detection of immune-mediated pathogenesis and early intervention in the disease course to improve the outcome.
Case reports
Case one
A 10-year-old boy, previously well and developmentally normal presented at a local hospital with a history of fever and focal motor seizures. He was hospitalized, and the initial cranial computed tomography scan revealed no significant abnormalities. His treatment started with intravenous (IV) ceftriaxone, vancomycin, and acyclovir for presumed meningoencephalitis. The seizures were treated with levetiracetam, reaching a 40 mg/kg/day dose. Complete blood count and biochemical analysis were normal. CSF was positive for HSV type 1 on PCR testing. He was treated with acyclovir for three weeks and discharged home in a good condition to the extent that he resumed his normal activities and attended school in the same week.
One week later, he was brought back to the hospital with altered sensorium, abnormal movements, irritability, and hallucinations. Physical examination revealed a child with a Glasgow Coma Scale of 9/15 (E4M4V1), oral dyskinesia, and choreoathetoid movements. He had left hemiparesis with a power of 3/5 on the left side with brisk deep tendon reflexes and extensor plantar response. He was commenced on IV immunoglobulin (IVIG) of 1 g/kg/day for two consecutive days and IV acyclovir treatment. Cerebral magnetic resonance imaging (MRI) showed bilateral hyperintensities involving temporal areas, limbic system, insular cortex, cingulate gyrus, and basifrontal region with bilateral asymmetry (right more than left). Electroencephalogram (EEG) showed diffuse delta slowing, not associated with clinical epileptic events or epileptiform abnormalities. CSF analysis revealed normal protein and glucose levels. Microscopic analysis of CSF revealed 90 leucocytes with 80% lymphocytes. Herpes PCR testing was negative. CSF sample detected at a titer of 1:10 using indirect immunofluorescence (a cell-based indirect immunofluorescence assay) was positive for anti-NMDAR autoantibodies. The patient was treated with a pulse IV methylprednisolone of 30 mg/kg/day for five days, followed by prednisolone of 2 mg/kg/day with slow tapering for 12 weeks. Trihexyphenidyl was started for the movement disorder. His condition worsened clinically with the suppressed level of consciousness and constant violent jerking abnormal movements, despite the immediate immunotherapy initiation. Subsequently, rituximab (375 mg/m2) was added to be taken weekly for four weeks for his anti-NMDAR encephalitis.
On day 48, he was discharged with constant choreoathetoid movements, expressive aphasia with left hemiparesis, irritability, and aggressive behavior. He had head control but could not sit or speak any words. Eight weeks later, he was readmitted with sleep disturbance and aggressive behavior in addition to swallowing difficulty. He required psychiatry evaluation, neurorehabilitation, gastroenterology consultation, and dietitian review. Six months later, he presented with a complete resolution of symptoms. He was back to baseline status with completely normal neurological examination including speech and cognitive functions. NMDAR antibodies were positive at three- and six-months follow-up with subsequent negative results at nine and 12 months with a titer of > 1:100 using indirect immunofluorescence. Follow-up MRI at 12 months showed interval partial improvement of the previous hyperintensities noted in the first MRI [Figure 1].
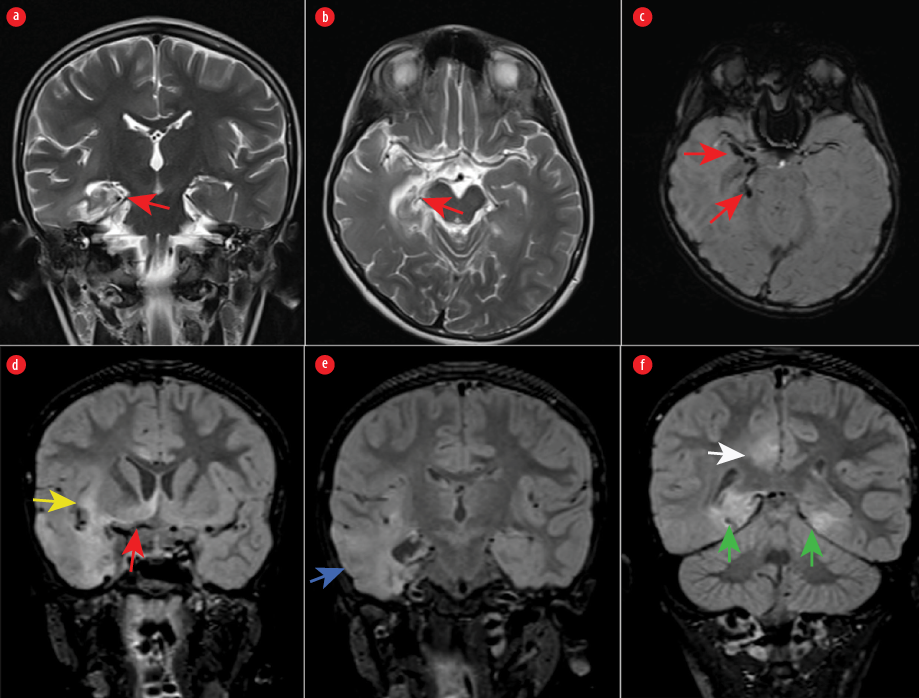 Figure 1: Atrophy and gliosis of the right hippocampus are seen in (a) coronal and (b) axial T2-weighted images (red arrows). (c) Susceptibility-weighted image showing old hemorrhage in the right mesial temporal lobe. (d, e, and f) Coronal fluid-attenuated inversion recovery images showing mild swelling and abnormal signal intensity in the inferior right frontal lobe (red arrow), right insula (yellow arrow), right temporal lobe with loss of gray-white matter differentiation (blue arrow), posterior medial temporal lobes bilaterally (green arrows), and right cingulate gyrus (white arrow).
Figure 1: Atrophy and gliosis of the right hippocampus are seen in (a) coronal and (b) axial T2-weighted images (red arrows). (c) Susceptibility-weighted image showing old hemorrhage in the right mesial temporal lobe. (d, e, and f) Coronal fluid-attenuated inversion recovery images showing mild swelling and abnormal signal intensity in the inferior right frontal lobe (red arrow), right insula (yellow arrow), right temporal lobe with loss of gray-white matter differentiation (blue arrow), posterior medial temporal lobes bilaterally (green arrows), and right cingulate gyrus (white arrow).
Case two
An eight-month-old infant presented with a history of fever and focal seizures. She was treated with ceftriaxone, vancomycin, and acyclovir, in addition to phenytoin, to control her seizures. A lumbar puncture was offered but refused by the parents. The initial blood workup was normal, with negative blood and urine cultures. Head CT showed hypodensities in bi-temporal areas. She was discharged in good condition after getting a 14-day course of acyclovir and antimicrobial therapy. However, she was readmitted after two weeks with irritability, altered sleep patterns, and involuntary limb and facial movements. Brain MRI revealed areas of increased signal intensity in the MRI of the frontal, hippocampal, thalamic, and temporal areas. EEG showed slow activity with sharp waves over the right frontal-temporal area. She was administered acyclovir intravenously. The parents were dissatisfied and left against medical advice. Her condition progressed, and she lost her ability to swallow the liquid and continued to have frequent seizures with choreoathetoid movements. Two months later, she was diagnosed with anti-NMDAR-autoimmune encephalitis in a pediatric hospital in the UAE. She received IVIG of 1 g/kg/day for two days, three doses of IV methylprednisolone pulse therapy, and three sessions of plasmapheresis. The steroid therapy was then continued orally for two months. In addition, she required multiple antiseizure medications consisting of levetiracetam, phenobarbitone, and clonazepam to control her epilepsy.
On subsequent follow-up visits, she continued to have uncontrolled seizures with impaired neurological development. Repeated anti-NMDAR was negative in serum. Her brain MRI showed a gliotic area in the right temporal lobe and T2 hyperintensity in periventricular white matter in the parietal and occipital region [Figure 2]. The EEG showed focal epileptiform discharges over the right hemisphere. Her current assessment, at five years old, revealed that she has a significant language delay, limited socialcommunication skills, repetitive behavior, and sensory sensitivity. She continues to have myoclonic seizures and focal motor seizures with impaired awareness. She has drug-refractory epilepsy despite being on the optimum dose of sodium valproate, levetiracetam, and clobazam.
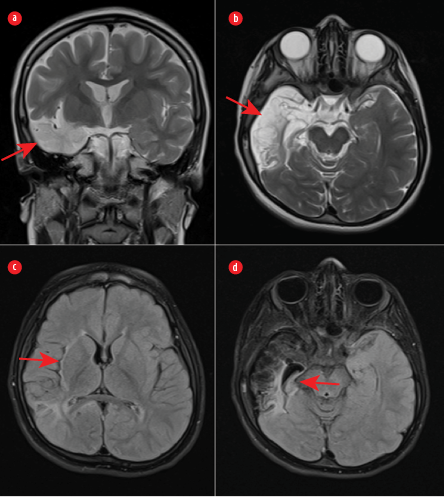 Figure 2: (a) Coronal and (b) axial T2-weighted images showing significant encephalomalacia of the right temporal lobe (red arrows). (c) An axial fluid-attenuated inversion recovery image demonstrating gliosis and volume loss in the right parietal lobe and right insula (red arrow). (d) Gliosis and significant atrophy of the right hippocampus (red arrow).
Figure 2: (a) Coronal and (b) axial T2-weighted images showing significant encephalomalacia of the right temporal lobe (red arrows). (c) An axial fluid-attenuated inversion recovery image demonstrating gliosis and volume loss in the right parietal lobe and right insula (red arrow). (d) Gliosis and significant atrophy of the right hippocampus (red arrow).
Discussion
HSV-induced anti-NMDAR encephalitis is known as autoimmune encephalitis, associated with the production of antibodies directed against the GLuN1 subunit of the NMDA receptor.6,7 We have presented two cases of early presentation of anti-NMDAR autoimmune encephalitis post-HSV-1 encephalitis in two pediatric age groups and two different outcomes. Though the etiology is unclear, it is hypothesized to be caused by the induction of synaptic antigens due to neuronal damage by viruses or by a molecular mimicry process (given the homology between NMDAR and HSV proteins).8,9 In a recent prospective cohort study it was found that in 51 patients with HSV encephalitis, 14 (27%) went on to develop autoimmune encephalitis. Among all the 14 participants, nine had antibodies against NMDAR, and five had antibodies against other neuronal proteins.10 Because of their similar presentations, autoimmune encephalitis and herpes virus reactivation should be considered in patients with worsening neurological impairments or emerging new neurological or psychiatric symptoms.11,12
The clinical manifestations often appear within three months after a successful course of anti-viral therapy.10 According to studies, presenting symptoms vary depending on the patient's age, with children exhibiting mostly neurological symptoms compared to adults, whose symptoms are more likely to emerge as behavioral and psychiatric problems.8,10,12 Additionally, variations in clinical presentation between pediatric groups were also discovered. A cohort study also showed that children under the age of four years frequently presented with frequent seizures, decreased level of consciousness, infantile spasms, or choreoathetosis with a worse outcome at one year, while those over the age of four frequently experienced changes in cognition and behavior that may be related to seizures.10 Choreoathetosis is the most encountered clinical feature in young children, in addition to drug-resistant epilepsy or status epilepticus.13–15 Most children and adults acquire the same clinical condition, which manifests with neurological and psychological symptoms, regardless of the presenting complaints.12
This report provides two examples of early anti-NMDAR AIE presentation within a month of HSV-1 encephalitis in two pediatric age groups (eight months and 10 years). Despite having a very similar time (one to two weeks) for the onset of illness following HSV encephalitis infection, the outcomes of the patients were vastly different. The fact that younger age at presentation tends to carry a worse prognosis and a delay in detection and treatment may account for the devastating outcome in the younger child. The first case highlights the value of prompt therapy with a successful outcome. The first case received first-line immunotherapy from the first day of his admission, and 72 hours later, second-line therapy commenced. However, the second case had significantly delayed treatment initiation and consequently developed significant brain damage. These manifested as uncontrollable seizures, global developmental delay (primarily speech and language), and autism spectrum disorder secondary to anti-NMDAR encephalitis. However, behavior and psychosis are challenging to assess in the younger age group. The older child presented with neurological, behavioral, and psychiatric symptoms as opposed to the infant who had more evident neurological symptoms. Infants and young children may experience significant neurological impairments and developmental disabilities, although older children and adults typically respond well to therapy with satisfactory results.8 There are no standard treatment protocols for managing autoimmune encephalitis. However, the consensus from systemic reviews is to initiate immunotherapy early, start first-line immunotherapy, and consider second-line immunomodulators for a better outcome and fewer relapses in case of no or poor response.7 In a study of 501 patients with NMDAR encephalitis resulting from various etiologies, the authors found that 53% of patients responded well to first-line medication and tumor excision and 81% of patients had a good prognosis after a 24-month follow-up.7
Corticosteroids, IVIG, and plasmapheresis are among the first-line treatments that can be administered separately or in combination. The most popular combination is corticosteroids and either IVIG or plasma exchange.16,17 Until the diagnosis of autoimmune encephalitis is confirmed, steroids should be deferred as they might aggravate infectious encephalitis, and their role in infectious encephalitis is controversial.11,18,19 In addition to their systemic manifestations, they can exacerbate the psychological symptoms linked to autoimmune encephalitis.7 IVIG is less expensive and more readily accessible for urgent therapy than plasma exchange.11 The second-line therapy needs to be commenced in case of little to no clinical response. The two alternatives are cyclophosphamide and rituximab.20 An improved safety profile for rituximab has been demonstrated.7 There is an option of long-term maintenance with prednisolone or steroid-sparing medications, such as mycophenolate mofetil and azathioprine, in cases where substantial neurological impairment persists and the patient’s response to treatment has been insufficient.
Conclusion
Clinical signs of HSV-induced anti-NMDAR encephalitis can arise months after a successful course of antiviral medication. The patient's age affects the symptoms, with youngsters more likely to have neurological symptoms than adults, whose symptoms are more likely to manifest as behavioral and psychiatric issues. Children with autoimmune encephalitis might have a better prognosis and outcome when the condition is identified early and immunotherapy is initiated promptly.
Disclosure
The authors declared no conflicts of interest. Written consent was obtained from the patient.
references
- 1. Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 2016 Jul;13(3):493-508.
- 2. Buechner S, Sixt GJ, Florio I. Herpes simplex virus (HSV)-1 encephalitis can induce chronic anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. J Neurosci Neurol Disord 2018;2(2):033-038.
- 3. Höftberger R, Armangue T, Leypoldt F, Graus F, Dalmau J. Clinical neuropathology practice guide 4-2013: post-herpes simplex encephalitis: N-methyl-Daspartate receptor antibodies are part of the problem. Clin Neuropathol 2013 Jul-Aug;32(4):251-254.
- 4. Kouba L, Alhosain D. A peculiar case of psychosis: anti-NMDAr encephalitis. Int J Emerg Med 2021;14(1):65.
- 5. Marques Macedo I, Gama Marques J. Catatonia secondary to anti-N-methyl-D-aspartate receptor (NMDAr) encephalitis: a review. CNS Spectr 2020 Aug;25(4):475-492.
- 6. Sahar N, Nurre AM, Simon RQ. Infectious trigger for autoimmune encephalitis: a case report and literature review. Case Rep Infect Dis 2019 Nov;2019:5731969.
- 7. Shin YW, Lee ST, Park KI, Jung KH, Jung KY, Lee SK, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord 2017 Aug;11:1756285617722347.
- 8. Alexopoulos H, Akrivou S, Mastroyanni S, Antonopoulou M, Dinopoulos A, Giorgi M, et al. Postherpes simplex encephalitis: a case series of viral-triggered autoimmunity, synaptic autoantibodies and response to therapy. Ther Adv Neurol Disord 2017;2018(11):1-8.
- 9. Ellul MA, Griffiths MJ, Iyer A, Avula S, Defres S, Baborie A, et al. Anti-N-methyl-d-aspartate receptor encephalitis in a young child with histological evidence on brain biopsy of coexistent herpes simplex virus type 1 infection. Pediatr Infect Dis J 2016 Mar;35(3):347-349.
- 10. Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al; Spanish Herpes Simplex Encephalitis Study Group. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018 Sep;17(9):760-772.
- 11. Hermetter C, Fazekas F, Hochmeister S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol 2018 Sep;9:706.
- 12. Erickson TA, Muscal E, Munoz FM, Lotze T, Hasbun R, Brown E, et al. Infectious and autoimmune causes of encephalitis in children. Pediatrics 2020 Jun;145(6):e20192543.
- 13. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013 Feb;12(2):157-165.
- 14. Armangue T, Moris G, Cantarín-Extremera V, Conde CE, Rostasy K, Erro ME, et al; Spanish Prospective Multicentric Study of Autoimmunity in Herpes Simplex Encephalitis. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 2015 Nov;85(20):1736-1743.
- 15. Li Q, Fu N, Han Y, Qin J. Pediatric autoimmune encephalitis and its relationship with infection. Pediatr Neurol 2021 Jul;120:27-32.
- 16. Lubarski K, Mania A, Michalak S, Osztynowicz K, Mazur-Melewska K, Figlerowicz M. The clinical spectrum of autoimmune-mediated neurological diseases in paediatric population. Brain Sci 2022 Apr;12(5):584.
- 17. Sechi E, Flanagan EP. Antibody-mediated autoimmune diseases of the CNS: challenges and approaches to diagnosis and management. Front Neurol 2021 Jul;12:673339.
- 18. Kumar R. Understanding and managing acute encephalitis. F1000Res 2020;9:F1000 Faculty Rev-60.
- 19. Hu S, Lan T, Bai R, Jiang S, Cai J, Ren L. HSV encephalitis triggered anti-NMDAR encephalitis: a case report. Neurol Sci 2021 Mar;42(3):857-861.
- 20. Bamford A, Crowe BH, Hacohen Y, Lin JP, Clarke A, Tudor-Williams G, et al. Pediatric herpes simplex virus encephalitis complicated by N-Methyl-D-Aspartate receptor antibody encephalitis. J Pediatric Infect Dis Soc 2015 Jun;4(2):e17-e21.