Psychosis is a complex disorder that effects the quality of life of patients and their family members.1 Being one of the most debilitating mental health conditions,2 the World Health Organization has included psychotic disorders among the global health challenges of the 21st century.3 Psychosis can be broadly defined as a loss of ego boundaries and a major impairment in reality testing.4 Early signs and symptoms tend to remain undiagnosed for a year or two, during which time the disease progresses.5 Early diagnosis is known to improve response to treatment, decrease recurrence, and improve the quality of life in patients.6,7 Delayed treatment tends to delay response to treatment, reduce recovery rate, and increase the risk of recurrence.8 The high treatment costs and the chronic nature of psychosis have led specialists to include psychosocial interventions such as family education, in adjutant with medication and patient care.9 The burden of caring for chronic mental patients tends to be borne by their families, usually parents. This has become an issue requiring raising the family’s knowledge levels on psychosis, ranging from behavioral to pharmacological care of the patient.10
Newer psychiatric medications are able to target the disease better with less side effects. Among these are the atypical antipsychotics, which are effective and have fewer side effects than conventional antipsychotics.11 However, they are not free of side effects that include extrapyramidal symptoms,12 hyperprolactinemia, sedation,13 and weight gain.12,14 These side effects can lead to a maladaptation between the patient and treatment.15 Among the atypical antipsychotic drugs prescribed to ambulatory patients are olanzapine and aripiprazole.
Olanzapine (sold under the trade name Zyprexa) is used to treat various types of psychoses. A recent clinical study reported that olanzapine caused more weight gain in black patients with schizophrenia than in their white counterparts, suggesting that ethnicity may also play a role.16 In a study by Wani et a,17 all the parameters of metabolic syndrome deteriorated in the olanzapine group.
Aripiprazole (trade name Abilify) is a second-generation antipsychotic and a potent 5HT2A antagonist.18 A study of children and adolescents who were prescribed aripiprazole to treat oppositional defiant disorder and conduct disorder showed positive effects in 60% of the subjects, but the initial dose had to be reduced due to vomiting and drowsiness.18
In clinical experience, the selection of atypical antipsychotics based on their potential side effects leads to treatment adherence in patients. Biological and environmental factors may affect the evidence for efficacy and side effects of these drugs. While both olanzapine and aripiprazole are considered effective in treating psychotic disorders and controlling the symptoms, the available evidence suggests that aripiprazole has a lower side effect than more complex drugs such as olanzapine. Wani et al,17 who compared the efficacies of both olanzapine and aripiprazole found that the aripiprazole significantly improved the patients’ metabolic syndromes as well as their psychiatric symptoms. A systematic review study by Ribeiro et al,19 showed that aripiprazole is as effective as other atypical antipsychotics, with less side effects. Another study found that aripiprazole was similar in effectiveness to risperidone, somewhat better than ziprasidone but less effective than olanzapine in treating psychiatric complaints.20 Aripiprazole also had overall less side effects than olanzapine and risperidone (such as weight gain, sleepiness, heart problems, shaking, and increased cholesterol levels), and patients were more likely to prefer it. However, ziprasidone was better than aripiprazole in dealing with restlessness.20
Ribeiro et al,19 a recent overview of systematic reviews on the efficacy and safety of aripiprazole found low to moderate quality of evidence, which requires further research in this area. Therefore, the aim of this study was to compare the efficacy and side effects of aripiprazole and olanzapine in patients with psychotic disorders.
Methods
The present study was a double-blind clinical trial. The study population included all patients age ranged 18–64 years with psychotic disorders who were referred to Farabi Hospital in Kermanshah, Iran during 2019–2020. The inclusion criteria were: (1) being diagnosed with a psychotic disorder based on clinical interview, Diagnostic and Statistical Manual-5, and International Classification of Sleep Disorders-3 criteria by a psychiatrist and (2) being prescribed aripiprazole or olanzapine by a psychiatrist as a treatment strategy for psychotic disorders. Exclusion criteria were: (1) taking other drugs with high sedative effects such as hypnotics concomitant with aripiprazole or olanzapine, (2) having a history of abuse/dependence on aripiprazole or olanzapine, and (3) having concomitant chronic illnesses such as diabetes, hypertension, rheumatism, or other chronic mental illnesses.
According to the type of study and using the data from Katshu et al,21 who reported the mean and SD of total sleep period before and after taking olanzapine as 380.43±113.80 min and 440.90±41.55 min, respectively, the minimum sample size required for our study was determined as 35 for each group at 95% CI and a power of 90%. Accounting for a 15% dropout, the final sample size was determined as 76 (38 each in the aripiprazole group and the olanzapine group). The subjects for the study were selected using convenience sampling from the patient population according to the inclusion and exclusion criteria.
Ethical permission was received from the Research Ethics Review Committee (Ref: IR.KUMS.REC.1399.376 dated July 14, 2020), the researcher was present among the participants and fully explained the study process. Then, a meeting was held with each participant separately, and after clearing any doubts and their interest in the research, a written consent was obtained from them.
First, all the participants underwent metabolic syndrome screening, including blood sugar, blood lipids, etc. Height and weight were measured to calculate body mass index (BMI). Then, one of the research team members, who was blind to the randomization of the two groups, completed the positive and negative symptoms questionnaire (PANSS) to all the patients.
The patients were then randomly divided into two groups of 38 each based on a table of random numbers. Each group was administered either olanzapine (n = 38) or aripiprazole (n = 38) but neither the participant nor the researcher knew which drug was administered. In the next step, one of the two drugs olanzapine and aripiprazole was prescribed to the patients by another psychiatrist for one month. The patients were also given a contact number so that they could call the psychiatrist in case of any problems. During the first month, patients were telephoned once a week and asked to narrate the side effects they experienced.
After the first month, the patients were called and asked to visit again. During this visit, the same psychiatrist re-examined the patients and prescribed them medication. The patients’ height and weight were taken again. The original researcher again completed the PANSS questionnaire and side effects checklist based on DSM-V. Since these drugs require about two months to demonstrate their effects, each patient was called again at the end of the second month of treatment, seen by the same psychiatrist, after which PANSS and side effects checklist were completed by the researcher for the third time.
It should be noted that in case of unwanted complications or metabolic syndrome at the end of the first or second month, the psychiatrist could change the medication based on the clinical condition of the patient. The assessing researcher, being blinded regarding the drugs prescribed, had no access to such information. To control confounding variables such as arbitrary use of other drugs or changing the dosage of the prescribed drug, the patients were called and reminded to avoid this.
After two months of follow-up, the data was analyzed using IBM SPSS Statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). The categorical and continuous variables characterizing the sample were described using descriptive statistics. Friedman and Wilcoxon tests were used to compare the pre- and post-treatment psychopathological scores. The normality status of PANSS scores in psychotic patients was investigated with the Shapiro-Wilk test. The Mann-Whitney test was used to compare the different variables between the two groups.
Results
The subjects of the study comprised N = 76 individuals diagnosed with psychosis, with a mean age of 35.7±9.0 years. Detailed demographic data is presented in Table 1.
Table 1: Demographic variables of the patients
(N = 76).
|
Sex
|
|
|
|
Male
|
65
|
85.5
|
|
Female
|
11
|
14.5
|
|
Marital status
|
|
|
|
Married
|
33
|
43.4
|
|
Single or divorced
|
43
|
56.6
|
|
Education
|
|
|
|
Under diploma
|
30
|
39.5
|
|
Diploma
|
34
|
44.7
|
|
Academic education
|
12
|
15.8
|
|
Occupation
|
|
|
|
Employed
|
27
|
35.5
|
|
Unemployed
|
49
|
64.5
|
|
Residence place
|
|
|
|
Urban
|
65
|
85.5
|
|
Rural
|
11
|
14.5
|
|
History of mental disorder
|
|
|
|
Yes
|
68
|
89.5
|
|
No
|
8
|
10.5
|
|
History of hospitalization
|
|
|
|
Yes
|
66
|
86.8
|
|
No
|
10
|
13.2
|
|
History of psychiatric drug use
|
|
Yes
|
70
|
92.1
|
|
No
|
6
|
7.9
|
|
Regular drug use
|
|
|
|
Yes
|
8
|
10.5
|
There were no significant differences between the olanzapine and aripiprazole groups regarding the distribution of sex, residence, history of mental disorder, history of hospitalization, history of psychiatric drug use, or history of regular drug use. Marital status, education, occupation, and age distribution were also similar between the groups. Shapiro-Wilk test was used to assess the normality status of PANSS scores. The Descriptive data and Shapiro-Wilk test results are given in Table 2.
Table 2: Normality status of scores of positive and negative syndrome scale in psychotic patients before, one month, and two months after treatment with olanzapine and aripiprazole (N = 76).
|
Negative symptoms
|
|
|
|
|
|
Before intervention
|
0.897
|
0.002
|
0830
|
< 0.001
|
|
After one month
|
0.808
|
< 0.001
|
0.687
|
< 0.001
|
|
After two months
|
0.765
|
< 0.001
|
0.781
|
< 0.001
|
|
Positive symptoms
|
|
|
|
|
|
Before intervention
|
0.896
|
0.002
|
0.879
|
0.001
|
|
After one month
|
0.806
|
< 0.001
|
0.656
|
< 0.001
|
|
After two months
|
0.708
|
< 0.001
|
0.831
|
< 0.001
|
|
Failure
|
|
|
|
|
|
Before intervention
|
0.945
|
0.059
|
0.957
|
0.157
|
|
After one month
|
0.936
|
0.030
|
0.910
|
0.005
|
|
After two months
|
0.612
|
< 0.001
|
0.731
|
< 0.001
|
|
Agitation
|
|
|
|
|
|
Before intervention
|
0.898
|
0.002
|
0.894
|
0.002
|
|
After one month
|
0.812
|
< 0.001
|
0.825
|
< 0.001
|
|
After two months
|
0.795
|
< 0.001
|
0.472
|
< 0.001
|
|
Anxiety and depression
|
|
|
|
|
|
Before intervention
|
0.895
|
0.002
|
0.925
|
0.014
|
|
After one month
|
0.902
|
0.003
|
0.883
|
0.001
|
|
After two months
|
0.727
|
< 0.001
|
0.808
|
< 0.001
|
|
Total score
|
|
|
|
|
|
Before intervention
|
0.954
|
0.125
|
0.973
|
0.493
|
|
After one month
|
0.898
|
0.002
|
0.936
|
0.030
|
Both olanzapine and aripiprazole patient groups experienced significant reductions in the mean scores of the negative symptom severity over the treatment period (p < 0.001) [Figure 1]. The effect sizes were satisfactory, at 0.496 for the olanzapine group and 0.615 for the aripiprazole group.
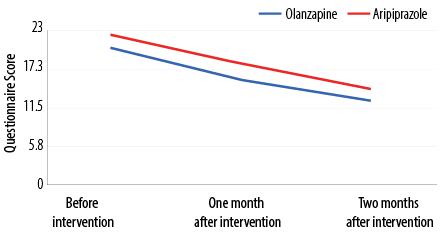 Figure 1: Comparative trend in change in the mean scores of the severity of the negative symptoms before and after two months of treatment with olanzapine and aripiprazole (N = 76).
Figure 1: Comparative trend in change in the mean scores of the severity of the negative symptoms before and after two months of treatment with olanzapine and aripiprazole (N = 76).
Both the olanzapine and aripiprazole groups experienced significant declines in the mean severity of their positive symptoms. There was a significant difference between the groups in their mean scores of positive symptom severity after one and two months of the intervention. Both the groups had a significant decreasing trend in the mean scores of failure, with no significant intergroup difference.
The mean scores of agitation for both groups significantly decreased over time, with no significant differences between the study groups. In the mean scores of anxiety and depression, both olanzapine and aripiprazole groups showed a significantly decreasing trend (p < 0.001), with no significant difference between the groups [Table 3].
Table 3: Comparison of the mean scores of anxiety and depression, body mass index (BMI), and waist circumference in the two study groups before, one month, and two months after the intervention.
|
The score of anxiety and depression
|
|
Olanzapine
|
8.8 ± 2.4
|
8.4 ± 2.7
|
8.4 ± 2.7
|
10.1
|
0.001
|
|
Aripiprazole
|
10.1 ± 2.4
|
8.4 ± 2.0
|
7.4 ± 1.3
|
0.001
|
0.001
|
|
Mann-Whitney
|
-2.400
|
-0.295
|
-0.457
|
|
|
|
p-value
|
0.015
|
0.768
|
0.645
|
|
|
|
BMI index
|
|
Olanzapine
|
23.2 ± 2.1
|
23.3. ± 2.05
|
23.4 ± 1.2
|
10.8
|
0.004
|
|
Aripiprazole
|
23.4 ± 2.8
|
23.4 ± 2.7
|
23.9 ± 2.9
|
6.2
|
0.043
|
|
Mann-Whitney
|
-0.312
|
-0.457
|
0.353
|
|
|
|
p-value
|
0.755
|
0.648
|
0.724
|
|
|
|
Waist circumference
|
|
Olanzapine
|
73.9 ± 11.6
|
73.9 ± 11.6
|
74.1 ± 11.9
|
8.3
|
0.016
|
|
Aripiprazole
|
75 ± 12.6
|
74.9 ± 12.5
|
75.1 ± 12.7
|
2.1
|
0.334
|
|
Mann-Whitney
|
-0.387
|
-0.351
|
-0.345
|
|
|
Friedman test revealed a significantly decreasing trend in the mean scores of the severity of positive and negative symptoms in both olanzapine and aripiprazole groups. Mann-Whitney test showed a significant difference in the mean scores of positive and negative symptoms between the study groups before the intervention and after one month and two months of the intervention. The above results were confirmed by repeated measures analysis. Table 3 also shows that the use of olanzapine and aripiprazole was associated with a significant increase in the BMI of our subjects during the study period. The mean waist circumference of the olanzapine group showed a significantly greater increase compared to the aripiprazole group (p = 0.016).
As shown in Table 4, olanzapine and aripiprazole intakes were associated with significant increases in the patients’ blood sugar over a two-month period, with a significant difference between the study groups.
Table 4: Comparison of mean blood sugar, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) in the studied groups before and two months after the intervention.
|
Blood sugar
|
|
|
|
|
|
Olanzapine
|
94.4 ± 8.3
|
94.4 ± 8.3
|
-2.6
|
0.005
|
|
Aripiprazole
|
93.3 ± 13.7
|
95.5 ± 9.3
|
-1.2
|
0.194
|
|
Mann-Whitney
|
-1.100
|
-0.937
|
|
|
|
p-value
|
0.265
|
0.330
|
|
|
|
LDL
|
|
|
|
|
|
Olanzapine
|
81.2 ± 23.2
|
86.5 ± 26.8
|
-2.6
|
0.009
|
|
Aripiprazole
|
74.5 ± 15.7
|
78.8 ± 16.5
|
-2.4
|
0.014
|
|
Mann-Whitney
|
-1.040
|
-0.110
|
|
|
|
p-value
|
0.298
|
0.236
|
|
|
|
HDL
|
|
|
|
|
|
Olanzapine
|
40.6 ± 6.1
|
44.4 ± 8.7
|
-4.1
|
0.008
|
|
Aripiprazole
|
42.6 ± 7.6
|
42.2 ± 6.8
|
-1.6
|
0.285
|
|
Mann-Whitney
|
-1.600
|
-0.074
|
|
|
Both olanzapine and aripiprazole groups had significant increases in triglyceride levels over the two treatment months, without significant intergroup differences [Figure 2].
 Figure 2: Trend of changes in mean triglyceride levels before and two months after intervention by study groups.
Figure 2: Trend of changes in mean triglyceride levels before and two months after intervention by study groups.
Table 4 shows that both olanzapine and aripiprazole caused significant increases in blood cholesterol levels of the patients over the two months of intervention. There was also a significant intergroup difference (p = 0.002). Further, both olanzapine and aripiprazole caused a significant increase in low-density lipoprotein cholesterol levels in patients, but without significant difference between the groups. Olanzapine increased high-density lipoprotein (HDL) cholesterol while aripiprazole decreased it (p = 0.008). In terms of sleep-related variables (sleep onset, sleep maintenance, sleep duration, and sleep quality), no significant differences or trends were found between the two groups.
Discussion
This study compared the profiles of psychiatric effectiveness and side effects of two new-generation antipsychotics, aripiprazole and olanzapine, in Iranian patients diagnosed with psychotic disorders. Olanzapine was more effective than aripiprazole in reducing the severity of negative and positive symptoms, which is consistent with a USA study.22 In general, both our groups showed a decreasing trend in positive and negative symptoms, but this trend was faster in the olanzapine group, in line with a Japanese study.23 The BMI of patients increased over time in both the groups, but this increase was greater in the aripiprazole group than in the olanzapine group, contradicting previous studies.22,24 Olanzapine increased HDL levels in patients, while aripiprazole decreased it, which is consistent with Henderson et al, findings.24 In our study, however, low-density lipoprotein levels increased in both groups while Henderson reported a decreasing trend.24 Overall cholesterol levels increased in both groups, more rapidly in the olanzapine group, against Henderson who saw falls in cholesterol levels.24
Wani et al,17 found that the replacement of olanzapine with aripiprazole reduced all the parameters of metabolic syndrome in the treated patients. As in Henderson's study, we observed a trend for triglyceride levels to slightly increase in both groups.24 The trend of changes in blood sugar also increased in both groups but more rapidly in the olanzapine group. The trend of changes in waist circumference was increasing in both groups but faster in the olanzapine group.
Due to the Covid-19 epidemic, this study was conducted with a limited sample size. Therefore, other studies with larger sample sizes are recommended. Future studies must be conducted with larger samples and longer follow-up periods. Delays in coordinating with several pharmacists to prepare drugs resulted in the loss of a large number of patients. In this study, we only considered pharmacological interventions. Other studies can also investigate the effectiveness of other non-pharmacological interventions. These findings will be useful for psychiatrists in Iran in selecting new-generation antipsychotics for treating patients with psychotic disorders.
Conclusion
The results of the present study showed that both olanzapine and aripiprazole treatments were associated with a reduction in positive and negative psychotic symptoms over a two-month treatment period. However, the reduction was faster with olanzapine. The BMI of patients increased over time in both groups and more so in the aripiprazole group. Agitation, anxiety, and depression, and failure scales decreased more slowly than the positive symptoms. Both drugs were associated with a rise in cholesterol levels, waist circumference, and blood sugar levels, but this trend was more rapid in the olanzapine group. Olanzapine increased HDL levels while aripiprazole decreased it.
Disclosure
The authors declared no conflicts of interest. The source of funding was received from the Vice-chancellor for Research and Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
References
- 1. Barlow DH, Durand VM. Abnormal psychology: an integrative approach. 5th ed. USA: Wadsworth; 2007.
- 2. Dyck DG, Hendryx MS, Short RA, Voss WD, McFarlane WR. Service use among patients with schizophrenia in psychoeducational multiple-family group treatment. Psychiatr Serv 2002 Jun;53(6):749-754.
- 3. Choi H, Hwang B, Kim S, Ko H, Kim S, Kim C. Clinical education in psychiatric mental health nursing: overcoming current challenges. Nurse Educ Today 2016 Apr; 39:109-115.
- 4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arjmand Publication; 2013. p. 129-163.
- 5. Norman RM, Malla AK. Duration of untreated psychosis: a critical examination of the concept and its importance. Psychol Med 2001 Apr;31(3):381-400.
- 6. Buckley PF, Correll CU, Miller AL. First-episode psychosis: a window of opportunity for best practices. CNS Spectr 2007 Sep;12(9)(Suppl 15):1-12, discussion 13-14, quiz 15-16.
- 7. Larsen TK, McGlashan TH, Moe LC. First-episode schizophrenia: I. Early course parameters. Schizophr Bull 1996;22(2):241-256.
- 8. Malla AK, Norman RM, Manchanda R, Ahmed MR, Scholten D, Harricharan R, et al. One year outcome in first episode psychosis: influence of DUP and other predictors. Schizophr Res 2002 Apr;54(3):231-242.
- 9. Miklovitz DJ. Family-focused treatment for adolescence with bipolar disorder. J Affect Disord 2009;82(Suppl 1):S113-S128.
- 10. Emami S, Kaikhavani S, Amirian K, Neyazi E. The effectiveness of family psychoeducation (atkinson and coia model) on mental health family members of patients with psychosis. Journal of Ilam University of Medical Sciences 2016;24(1):8-17.
- 11. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr 2014 Oct;11:3-7.
- 12. Kane JM. Addressing side effects from antipsychotic treatment in schizophrenia. J Clin Psychiatry 2011 Feb;72(2):e07.
- 13. Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull 2015 Jun;114(1):169-179.
- 14. De Hert M, Yu W, Detraux J, Sweers K, van Winkel R, Correll CU. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs 2012 Sep;26(9):733-759.
- 15. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol 2017 Apr;37(2):138-147.
- 16. Stauffer VL, Sniadecki JL, Piezer KW, Gatz J, Kollack-Walker S, Hoffmann VP, et al. Impact of race on efficacy and safety during treatment with olanzapine in schizophrenia, schizophreniform or schizoaffective disorder. BMC Psychiatry 2010 Nov;10(1):89.
- 17. Wani RA, Dar MA, Chandel RK, Rather YH, Haq I, Hussain A, et al. Effects of switching from olanzapine to aripiprazole on the metabolic profiles of patients with schizophrenia and metabolic syndrome: a double-blind, randomized, open-label study. Neuropsychiatr Dis Treat 2015 Mar;11:685-693.
- 18. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock's synopsis of psychiatry: behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015.
- 19. Ribeiro EL, de Mendonça Lima T, Vieira ME, Storpirtis S, Aguiar PM. Efficacy and safety of aripiprazole for the treatment of schizophrenia: an overview of systematic reviews. Eur J Clin Pharmacol 2018 Oct;74(10):1215-1233.
- 20. Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, El-Sayeh HG, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2009;(4):CD006569.
- 21. Katshu MZ, Sarkar S, Nizamie SH. Effect of olanzapine on clinical and polysomnography profiles in patients with schizophrenia. Schizophr Res Treatment 2018 Feb;2018:3968015.
- 22. Citrome L. A review of aripiprazole in the treatment of patients with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat 2006 Dec;2(4):427-443.
- 23. Kishi T, Matsuda Y, Matsunaga S, Iwata N. Aripiprazole for the management of schizophrenia in the Japanese population: a systematic review and meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat 2015 Feb; 11:419-434.
- 24. Henderson DC, Fan X, Copeland PM, Sharma B, Borba CP, Boxill R, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol 2009 Apr;29(2):165-169.