Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzymopathy, affecting up to 400 million individuals globally. The highest prevalence is in Africa, Southern Europe, and Asia, especially the Middle East and Southeast Asia. The distribution of this disease mirrors that of malaria, which gave rise to the notion that G6PD deficiency confers some protection against malaria. The inheritance of G6PD deficiency follows an X-linked pattern, hence males can be either hemizygous normal or hemizygous deficient, whereas females may be homozygous normal, homozygous deficient, or heterozygous.1
G6PD is an enzyme that catalyzes the first reaction in the pentose phosphate pathway. This pathway is crucial in providing pentose sugars from glucose for glycolysis and generating nicotinamide adenine dinucleotide phosphate (NADPH), which provides reducing power to red blood cells (RBCs).2 G6PD breaks down glucose by catalyzing the oxidation of β-D-glucose-6-phosphate to D-glucono-1,5-lactone 6-phosphate. The byproduct of this reaction is NADPH. D-glucono-1,5 lactone-6-phosphate is then hydrolyzed forming 6-phosphogluconate, which will then be decarboxylated by 6-phosphogluconate dehydrogenase enzyme. This reaction will yield the five-carbon molecular ribulose 5-phosphate, which is a precursor of DNA, RNA, and ATP and concomitantly generates another NADPH molecule.3 In RBCs, the pentose phosphate pathway is the only source of NADPH due to the absence of mitochondria. NADPH is crucial in protecting the cells against reactive oxygen species (ROS). NADPH participates in the glutathione pathway by donating an electron to glutathione dimers, becoming oxidized glutathione/glutathione disulfide. This reaction is catalyzed by the glutathione reductase enzyme which produces two reduced glutathione monomers, which are the first line of defense against ROS.2 NADPH is also needed to reduce glutathione disulfide and the sulfhydryl groups of some necessary proteins which protect against oxidative stress. If this protection against ROS is absent, RBCs can undergo oxidative hemolysis.3
Since males are hemizygous for the G6PD gene, they can be frankly G6PD deficient or have normal levels of G6PD. Females, on the other hand, have two copies of the G6PD gene on each X chromosome, so they can have normal gene expression or be heterozygous. In places where the frequency of the G6PD deficient allele is very high, it is not rare to find homozygous females. Because of lyonization or X-chromosome inactivation, heterozygous females are genetic mosaics. Their abnormal cells can be G6PD deficient or G6PD intermediate, rendering these individuals susceptible to oxidative stress and related complications.2
G6PD deficiency can be diagnosed using quantitative or qualitative/semi-quantitative tests. The most extensively used qualitative test is the fluorescent spot test (FST). For decades, FST has been the standard screening test for G6PD deficiency in Malaysia. It is much cheaper than other quantitative G6PD enzyme assays and gives reasonably reliable binary results (deficient or normal). However, several studies have shown that the FST may be missing many cases with intermediate levels of G6PD.4 This is potentially detrimental because even individuals with intermediate levels of G6PD can have hemolytic crises. On the other hand, several quantitative tests are available to measure G6PD activity such as the spectrophotometric assay (the current gold standard), and point-of-care quantitative assay such as biosensor 1. Quantitative enzyme assays can quantify the amount of G6PD activity either by normalization of hemoglobin or RBC count.5
The objectives of this study were to compare the performance of FST with CareStart Bioensor 1 and to evaluate the difference in the estimated prevalence of G6PD deficiency in neonates using each method.
Methods
A cross-sectional study was conducted among neonates born in Hospital Universiti Sains Malaysia, Kelantan, Malaysia between June and December 2020. This study was carried out upon receiving the ethical approval of the Human Research Ethics Committee of Universiti Sains Malaysia (Ref: USM/JEPeM/19120878). Random sampling was performed, taking only samples that fulfilled the inclusion and exclusion criteria. We included all cord blood samples sent within the study frame. Excluded from the study were all clotted blood samples and samples taken from neonates with severe congenital anomalies or severe intrauterine growth restriction. Two milliliters of cord blood were taken in EDTA bottles during delivery for quantitative enzyme activity measurement using CareStart Biosensor 1. For FST, one drop of cord blood was directly placed on a piece of filter paper and allowed to dry completely before placing it in a biohazard bag. The sample was sent to the laboratory within four hours of collection. All FST samples were analyzed within 24 hours of receipt.
The FST was performed using Atlas Medical G6PD Qualitative Kit that employs long-wave ultraviolet (UV) light. The principle behind the test is that in a normal patient, NADPH generated by the G6PD enzyme present in a lysate of blood cells fluoresces under longwave UV light. In G6PD deficient patients, insufficient NADPH is produced, resulting in low or no fluorescence. For the test, 100 μL of the working mix was pipetted into each sample and control tube. The samples were mixed well with the working mix and incubated at 37 °C in a drying oven for 30 minutes. After drying the spots were observed under fluorescent UV light of 365 nm wavelength using a UV viewing box. The results were recorded and validated. The sample was reported as ‘normal’ if the spot fluoresced under UV light, ‘intermediate’ if the fluorescence was slight, and ‘deficient’ if there was no fluorescence.
The quantitative assay was performed using CareStart Biosensor 1 (Wells Bio Inc. Korea). It employs an electrochemical method to measure enzyme activity in a sample. It measures the electron transfer from NADPH during conversion to NADPH+. The magnitude of the electric current produced is directly proportional to the level of G6PD activity in the blood sample. Before this test was performed, EDTA tubes filled with cord blood samples were arranged on test tube racks for 30 minutes to let the samples reach room temperature. The hemoglobin (Hb) strip and G6PD strip were inserted at the designated spots on the analyzer and 20 μL of blood was pipetted on each strip. Reading from the machine was then recorded on a worksheet.
For the two methods (FST and biosensor 1) we used the manufacturers’ reference ranges. The cut-off values, as verified by our inhouse laboratory using the transference method recommended by the Clinical and Laboratory Standard Institute,6 were as follows: deficient: < 30% of mean normal G6PD activity (< 2.8 U/g Hb), intermediate: 30–60% of mean normal G6PD activity (2.8–5.6 U/g Hb), normal: > 60% of mean normal G6PD activity (> 5.6 U/g Hb).
Data was analyzed using IBM SPSS Statistics (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and prevalence were calculated for FST (we did not perform the gold standard assay to calculate these values for biosensor). Cohen’s kappa (κ) analysis was conducted to assess the agreement between the two methods. Since the clinical implications for both deficient and intermediate groups were the same, both groups were classified as deficient. The paired McNemar test was used to analyze the difference in the prevalence of G6PD deficiency as assessed by FST and biosensor 1. P-value < 0.050 was considered statistically significant.
Results
Cord blood samples from 455 neonates were used in this study. There were 238 (52.3%) female and 217 (47.7%) male babies. The vast majority (443; 97.4%) were of Malay ethnicity followed by Thai (0.9%), Arab (0.7%), Rohingya (0.7%), and Chinese (0.4%). Term neonates yielded 81.5% and preterm neonates yielded 18.5% of samples.
When compared with biosensor 1, at a 30% cut-off value of G6PD activity, the FST had high sensitivity (91.0%; 95% CI: 57.0–100), high specificity (97.0%; 95% CI: 95.0–98.0), and high NPV (99.8%; 95% CI: 98.0–99.0). However, the PPV was low at only 43.5% (95% CI: 24.0–65.0). The prevalence of G6PD deficiency was 2.4% when measured by FST at a 30% cut-off. In contrast, at the 60% cut-off value, the sensitivity dropped to 29.0% (95% CI: 19.0–40.0), the NPV dropped to 86.8% (95% CI: 83.0-89.0), and the PPV increased to 100% (95% CI: 98.0–100). The prevalence of G6PD deficiency when measured by FST at 60% cut-off was only 1.8%. Cohen’s κ showed only fair agreement between the two methods, κ = 0.21, p < 0.001 [Table 1].
Table 1: Performance of fluorescent spot test at specific G6PD cut-off values.
|
30
|
< 2.8
|
91.0 (57.0–100)
|
97.0 (95.0–98.0)
|
43.5 (24.0–65.0)
|
99.8 (98.0–99.0)
|
2.4 (1.2–4.4)
|
G6PD: glucose-6-phosphate dehydrogenase.
The overall prevalence of G6PD deficiency—comprising neonates with both deficient and intermediate levels of G6PD—was 5.1% as determined by FST and 17.8% by the biosensor 1 method. The overall difference between the two methods remained significant across groups stratified by gender and gestational age (p < 0.001) [Table 2].
The prevalence of G6PD deficiency, divided into deficient, intermediate, and normal levels, differed when stratified by gender and gestational age [Table 3].
Table 2: Differences in prevalence of G6PD deficiency across neonatal groups.
|
Male
|
218
|
Normal
|
185
|
16
|
< 0.001
|
|
|
Deficient
|
0
|
17
|
|
|
Female
|
237
|
Normal
|
189
|
42
|
< 0.001
|
|
|
Deficient
|
0
|
6
|
|
|
Term
|
371
|
Normal
|
308
|
44
|
< 0.001
|
|
|
Deficient
|
0
|
19
|
|
|
Preterm
|
84
|
Normal
|
66
|
14
|
< 0.001
|
G6PD: glucose-6-phosphate dehydrogenase; FST: fluorescent spot test; biosensor 1: CareStart Biosensor 1.
Table 3: Prevalence of G6PD deficiency stratified by sex and gestational age at birth.
|
Male
|
FST
|
16 (7.3)
|
1 (0.5)
|
201 (92.2)
|
|
Biosensor 1
|
9 (4.1)
|
24 (11.0)
|
185 (84.9)
|
|
|
|
|
|
|
Female
|
FST
|
4 (1.7)
|
2 (0.8)
|
231 (97.5)
|
|
Biosensor 1
|
0 (0.0)
|
48 (20.3)
|
189 (79.7)
|
|
Term
|
FST
|
16 (4.3)
|
3 (0.8)
|
352 (94.9)
|
|
Biosensor 1
|
8 (2.2)
|
55 (14.8)
|
308 (83.0)
|
|
|
|
|
|
|
Preterm
|
FST
|
4 (4.8)
|
0 (0.0)
|
80 (95.2)
|
G6PD: glucose-6-phosphate dehydrogenase; FST: fluorescent spot test; biosensor 1: CareStart Biosensor 1.
Figure 1 depicts the distribution of G6PD enzyme level (U/g Hb) measured by biosensor 1 according to FST status while Figure 2 shows the distribution of G6PD level across gender groups.
 G6PD: glucose-6-phosphate dehydrogenase.
G6PD: glucose-6-phosphate dehydrogenase.
Figure 1: Distribution of G6PD level according to fluorescent spot test status.
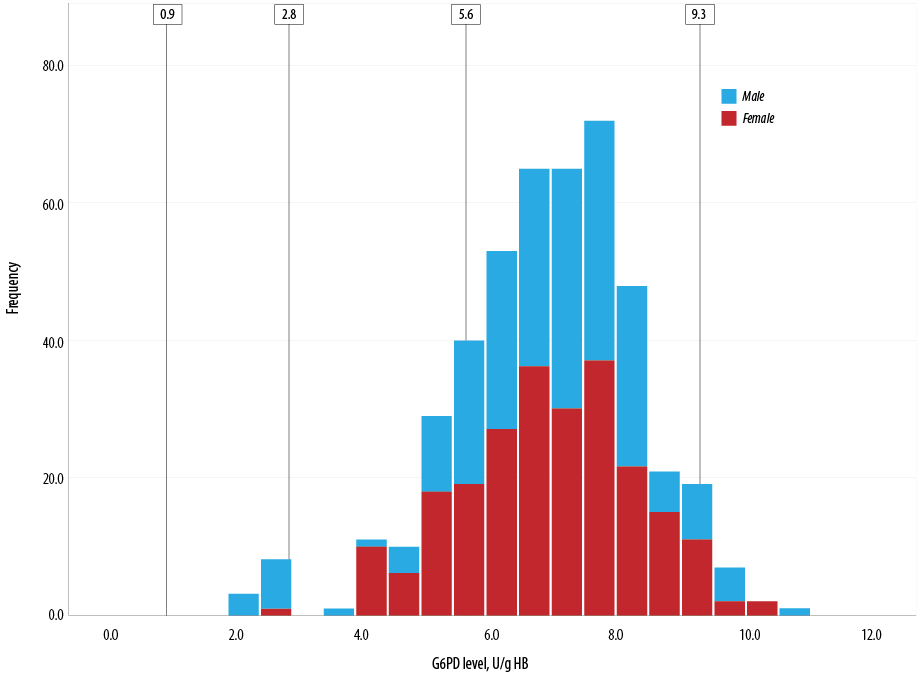 Figure 2: Distribution of glucose-6-phosphate dehydrogenase (G6PD) level across gender groups.
Figure 2: Distribution of glucose-6-phosphate dehydrogenase (G6PD) level across gender groups.
Levels 0.9 U/g Hb is < 10% of normal G6PD activity, 2.8 U/g Hb is < 30% of normal G6PD activity, and 5.6 U/g Hb is the 60% cut-off value for normal G6PD while 9.3 U/g Hb is 100% G6PD activity.
Discussion
This study was conducted in Kelantan, a state in the Northeast region of peninsular Malaysia. It borders Southern Thailand and has a population of 1.4 million people. The vast majority are ethnically Malay (95%), followed by Thai (3%), Chinese (1.9%), and other (0.1%) ethnicities.7 Our sample also had a similar demographic distribution.
The diagnostic performances of FST in this study are outlined in Table 1. At a lower G6PD threshold (< 30% activity), the FST showed high sensitivity and specificity. However, when the threshold was raised to < 60% of activity to include individuals with intermediate or partial G6PD deficiency, the sensitivity reduced drastically to 29.0% while maintaining high specificity. These findings were consistent with previous studies. In a study by Henriques et al,8 the sensitivity of FST was 100% at < 30% activity level but decreased to 65% at < 70% activity level, while in a study by Thielemans et al9, the FST sensitivity was 91.4% at < 30% activity with a specificity of 99.9%. It is interesting that in the current study, the reduction of sensitivity at < 60% threshold was more drastic than in the study by Henriques et al.8 This might be due to difference in patient ages, as in the aforementioned study population consisted of participants aged ≥ 4 years. It can be theorized then that G6PD levels in neonate cord blood have a narrower distribution range than in children and adults. At both cut-off values, FST had high specificity implying that false positives with FST were exceedingly rare.
The PPV for FST at < 30% cut-off value was low (43.5%), which was an appreciable difference from previous studies. Thielemens et al,9 found that the PPV for FST when used in newborn cord blood samples was 97.7% (95%CI: 96.9–98.5). On the other hand, LaRue et al,10 found that the PPV was 72.0% (95% CI: 50.6–87.9) at 30% cut-off value.In our study, samples diagnosed by FST as G6PD deficient were mainly in the intermediate group when classified according to their G6PD enzyme activity by biosensor 1. This could be explained by the homogeneity of our study sample, 97.4% of whom had Malay ethnicity.
The overall prevalence of G6PD deficiency as measured by FST was 5.1% in comparison with biosensor 1 at 17.8% (p < 0.001). The higher prevalence of G6PD deficiency when tested using quantitative assay was also seen in other studies.4,8 Ainoon et al,4 found that the prevalence of G6PD deficiency was 9.8% when using a spectrophotometer for quantitative G6PD enzyme assay, while FST gave 1.3%. They used a cut-off point of < 20% for G6PD deficiency, which may explain the lower prevalence levels.
This study showed 5.1% G6PD deficiency prevalence in the Kelantanese population, which is higher than the global levels. Southern Thailand also has a high prevalence of G6PD deficiency.11 Ninokata et al,11 found similarly high levels of G6PD deficiency among the Moken (15.4%) and Thai (15.5%) ethnic groups. Amongst the Moken, the G6PD variants were G6PD Mahidol, G6PD Gaohe, and G6PD Viangchan. Interestingly, G6PD Mahidol and G6PD Viangchan are also found in ethnic Malays. This phenomenon adds to the genetic makeup of the Kelantanese population who live near the Thailand border due to the region’s history of cross-border immigration and marriages.
In our study, biosensor 1 detected a significantly higher proportion of neonates with intermediate G6PD deficiency (15.8%) than FST (0.7%) did. This yield difference was more marked among female neonates. In males, FST was able to detect 16 (7.3%)neonates with G6PD deficiency and one (0.5%)neonate with intermediate G6PD level. In female neonates, FST was able to detect four (1.7%) with G6PD deficiency and two (0.8%) with intermediate G6PD levels. This is in stark contrast with biosensor 1 which was able to detect 48 (20.3%) female neonates as having intermediate G6PD levels.
As mentioned earlier, due to the X-chromosome-linked nature of G6PD inheritance, females can have normal gene expression, be heterozygous or rarely homozygous for a mutation or compound heterozygous for two mutations on the G6PD gene. Females inherit two copies of the alleles on X chromosomes, however, due to X-inactivation, the individual RBCs in heterozygous females have G6PD enzyme expression from either the normal allele or the mutated allele, which will bring forth two distinct populations of RBCs, one containing normal G6PD level and the other with decreased G6PD expression. The total G6PD activity of a heterozygous female is the relative ratio of the two RBC populations.12 Consequently, some heterozygous females may have ratios that have a high proportion of RBCs with normal G6PD enzyme levels while others may have a high proportion of RBCs with decreased G6PD enzyme levels. This implies that many heterozygous females may have enzyme levels between 30–60%, indicating intermediate G6PD deficiency.
When using a cut-off < 60% of normal mean activity as an intermediate level, more female neonates in our study were detected by biosensor 1. Biosensor 1 also found significantly higher prevalence of intermediate G6PD deficiency among preterm neonates (20.2%) compared to term neonates (14.8%).
The Hardy-Weinberg equilibrium can be used to predict the genotype distribution of two alleles in a population. However, clinically, it is the phenotypic distribution of G6PD manifestation that is usually used to assess the prevalence of G6PD deficiency in a population. G6PD alleles can have different distributions of G6PD activity in heterozygous females. This implies that the prevalence of G6PD deficit among heterozygous females in a population can be skewed towards higher or lower compared to the general population. This does not significantly affect the male distribution, however, as males are homozygous deficient or homozygous normal.12 In males, the distinction between G6PD deficient and G6PD normal is more marked, while in females, the distribution is more continuous. This meant that males who are G6PD deficient predominantly have values < 30% of normal G6PD activity while heterozygous females have values ranging from 30–60%.
There are many clinical implications of misclassifying females with intermediate G6PD levels as having normal enzyme activity. These go beyond the neonatal period. In the neonatal period, G6PD deficient and G6PD intermediate infants are at higher risk of neonatal jaundice that can lead to kernicterus. Some countries require all G6PD-deficient neonates to be observed for the first five days of birth for signs and symptoms of neonatal jaundice. However, if a female neonate has been misclassified as having a normal G6PD level by FST, then she is more at risk of having neonatal jaundice as she will not go through the same vigilant observation performed for G6PD deficient neonates.
Beyond the neonatal period, heterozygous females are at risk of developing hemolysis after exposure to oxidative challenges. This is because hemolysis is not only affected just by the level of G6PD enzyme in the RBCs. Instead, other important factors such as the affinity of the existing G6PD enzyme for the substrate, the regeneration of new RBCs after a menstrual cycle, and other important environmental factors such as the ingestion of fava beans all play different roles in the development of hemolysis in these individuals.13 Also, it has been shown that G6PD measurement in a neonate can differ from subsequent measurements, which contributes to the susceptibility of the RBC populations to oxidative stress.1,4,9
The gold standard for G6PD quantitative assay is the spectrophotometer. However, this test is laborious, requires adequately trained laboratory personnel, and dedicated laboratory equipment which is expensive to buy and operate.14 The results are also time-consuming. This renders spectrophotometry unsuitable for a field test, for example, in guiding the decision for malaria prophylaxis.8 The advantages of using biosensor 1 as a point-of-care test include lower costs, relative ease of operation by minimally trained staff, and faster result availability.
Conclusion
Though FST is being widely used in Malaysia for G6PD deficiency screening, it has certain drawbacks. The low sensitivity of FST at a 60% cut-off value is undesirable as it leads to the misclassification of heterozygous females as having normal G6PD levels. However, at a 30% cut-off value, its sensitivity is acceptable to correctly predict individuals having G6PD deficiency. This study has demonstrated the differences in the analytical performance of FST and biosensor 1, while also illustrating the difference in the prevalence of G6PD deficiency in neonates as assessed by each method.
Disclosure
The authors declared no conflicts of interest. This study was funded by USM grant 1001/PPSP/8070011.
Acknowledgements
We would like to thank Ms. Noor Suhana Azila binti Mat Yusoff, senior medical laboratory technologist for her technical support, and all labor room personnel for their assistance with
sample collection.
references
- 1. van den Broek L, Heylen E, van den Akker M. Glucose-6-phosphate dehydrogenase deficiency: not exclusively in males. Clin Case Rep 2016 Oct;4(12):1135-1137.
- 2. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008 Jan;371(9606):64-74.
- 3. Gómez-Manzo S, Marcial-Quino J, Vanoye-Carlo A, Serrano-Posada H, Ortega-Cuellar D, Abigail González-Valdez A, et al. Functional and biochemical analysis of glucose-6-phosphate dehydrogenase (G6PD) variants: elucidating the molecular basis of G6PD deficiency. Int J Mol Sci 2016;17(12):2069.
- 4. Ainoon O, Alawiyah A, Yu YH, Cheong SK, Hamidah NH, Boo NY, et al. Semiquantitative screening test for G6PD deficiency detects severe deficiency but misses a substantial proportion of partially-deficient females. Southeast Asian J Trop Med Public Health 2003 Jun;34(2):405-414.
- 5. Roper D, Layton M, Rees D, Lambert C, Vulliamy T, De la Salle B, et al; British Society for Haematology. Laboratory diagnosis of G6PD deficiency. A British society for haematology guideline. Br J Haematol 2020 Apr;189(1):24-38.
- 6. Henny J, Vassault A, Boursier G, Vukasovic I, Mesko Brguljan P, Lohmander M, et al; Working Group Accreditation and ISO/CEN standards (WG-A/ISO) of the EFLM. Recommendation for the review of biological reference intervals in medical laboratories. Clin Chem Lab Med 2016 Dec;54(12):1893-1900.
- 7. Mohd Rashid Z, Yusuff NA. Chinese and Siamese cultures in Malay Muslims environment. 2010 [cited 2021 April 25]. Available from: http://umkeprints.umk.edu.my/6124/.
- 8. Henriques G, Phommasone K, Tripura R, Peto TJ, Raut S, Snethlage C, et al. Comparison of glucose-6 phosphate dehydrogenase status by fluorescent spot test and rapid diagnostic test in Lao PDR and Cambodia. Malar J 2018 Jun;17(1):243.
- 9. Thielemans L, Gornsawun G, Hanboonkunupakarn B, Paw MK, Porn P, Moo PK, et al. Diagnostic performances of the fluorescent spot test for G6PD deficiency in newborns along the Thailand-Myanmar border: a cohort study. Wellcome Open Res 2018 Jan;3:1.
- 10. LaRue N, Kahn M, Murray M, Leader BT, Bansil P, McGray S, et al. Comparison of quantitative and qualitative tests for glucose-6-phosphate dehydrogenase deficiency. Am J Trop Med Hyg 2014 Oct;91(4):854-861.
- 11. Ninokata A, Kimura R, Samakkarn U, Settheetham-Ishida W, Ishida T. Coexistence of five G6PD variants indicates ethnic complexity of Phuket islanders, Southern Thailand. J Hum Genet 2006;51(5):424-428.
- 12. Domingo GJ, Advani N, Satyagraha AW, Sibley CH, Rowley E, Kalnoky M, et al. Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: challenges and opportunities. Int Health 2019 Jan;11(1):7-14.
- 13. Reclos GJ, Hatzidakis CJ, Schulpis KH. Glucose-6-phosphate dehydrogenase deficiency neonatal screening: preliminary evidence that a high percentage of partially deficient female neonates are missed during routine screening. J Med Screen 2000;7(1):46-51.
- 14. Pengboon P, Thamwarokun A, Changsri K, Kaset C, Chomean S. Evaluation of quantitative biosensor for glucose-6-phosphate dehydrogenase activity detection. PloS one 2019 Dec 20;14(12):e0226927.