The coinfection of diabetes mellitus (DM) and tuberculosis (TB) has emerged as a global health problem. People infected with DM are three times more likely to get TB.1 The World Health Organization has highlighted DM as a neglected, significant, and remerging TB risk factor.2 Occurrences of coinfection are documented in low- and middle-income countries where TB is endemic, and cases are rising.3
Globally, 15% of TB patients have DM, while the prevalence of DM among TB patients ranges from 5% to over 50% in Asian nations.4 In Middle Eastern countries, the prevalence of DM among TB patients varies widely, from 4.2% in Iran to 60% in Yemen. Studies of African countries show significant variations in the prevalence of DM among TB patients (3.35–16.4%). In Gulf Cooperation Council countries, few indirect investigations into the prevalence of DM among TB patients have been undertaken. The prevalence for Saudi Arabia, Kuwait, and Qatar, range from 14–26% (1989–2009), 29.8–35% (1996–2005), and 5–25.5% (1996–2009).5
In Oman, the prevalence of DM is 15.7% of the total population.6 However, the prevalence of type 2 DM (T2DM) in Muscat was estimated at 16.6% affecting 14.5% of Omani citizens and 18.8% of non-Omani citizens, making it a leading cause of morbidity and mortality.7 In contrast, the prevalence of TB (all types) in Oman has dropped, with the prevalence of bacteriologically confirmed pulmonary TB decreasing from 19.61 per 100 000 persons in 1981 to 3.5 per 100 000 persons in 2020.6 Therefore, Oman’s high prevalence of DM triples the risk of developing TB and result in adverse treatment outcomes. This emphasizes the need to implement bidirectional screening programs to improve outcomes.
According to studies on TB-DM, being male, over 45 years old, normal or overweight, having a low educational level, and living in rural areas are all predictive factors for TB-DM.8–13 In 2015, a systematic review identified 59 papers on DM and TB from Middle Eastern nations that indicated no difference between the sexes, with few studies indicating that males are more likely to be affected.14,15 Additionally, TB-DM patients are associated with age (50±10 years), obesity, and HIV coinfection. Pulmonary TB is the most common form of TB in almost all studies of comorbid patients. These patients are more susceptible to morbidity and mortality, including treatment failure, TB medication resistance, and relapses compared to TB patients without DM. 5
More research is needed to understand the epidemiology of DM among TB patients in Oman. Determinants can vary by place, even within the same age group, sex, and ethnicity. There is no national study on diabetes and its associated factors among TB patients in Oman. We aim for early detection, bidirectional screening, effective clinical management, and prevention of DM in adult TB patients. This study will be the first to evaluate TB-DM prevalence in Oman and the Arab Gulf region. Healthcare providers and policymakers will be enlightened about strategies that could help protect those most at risk of developing untreatable TB-DM complications. By using the results of this study, policymakers can develop coordinated and effective intervention programs for two comorbidities, thereby minimizing overload on the healthcare system and minimizing the burden of TB-DM.
The objectives of this study are to assess the prevalence of DM in adult TB patients and to identify its associated factors in Muscat from 2017–2020.
Methods
An analytical cross-sectional study was conducted to determine the prevalence of DM among TB patients and its associated factors in Muscat, Oman.
The study was conducted in Muscat governorate between 1 January 2017 and 31 December 2020. It is the capital city of Oman and is populated by 1 365 784 people, of which 60.7% are non-Omani citizens and 39.3% are Omani. The governorate is composed of six provinces: Al Amerat, Bowsher, Muscat, Muttrah, A'Seeb, and Quriyat.16 The Omani Ministry of Health considered ages > 13 years old as adults and therefore their treatment and follow-up are in the adult departments in the health institutions.
TB diagnoses are reported electronically through the health system (Al-Shifa 3+). TB patients were screened for DM in the TB clinic using random blood sugar. If the patient’s random blood sugar was abnormal, fasting blood glucose (overnight fasting) and glycated hemoglobin A1c were performed upon admission. If fasting blood glucose ≥ 126 mg/dL (≥ 7 mmol/L), patients were referred to the DM clinic for diagnosis confirmation, follow-up, and therapy.17 Sometimes, DM patients are diagnosed unintentionally at TB clinics. Some patients were already diagnosed with T2DM and followed-up in primary healthcare institutions. Therefore, they were known T2DM patients when infected with TB.
Al-Shifa 3+ system is perhaps the most obvious milestone of the digital health system in the Sultanate, which manages medical records at health establishments. It is accessible to medical staff and administrators. The detailed data of each patient is entered by authorized practicing doctors. TB e-notification form was reviewed and confirmed by the central authority in the Ministry of Health. Al-Shifa 3+ is a comprehensive and adaptable online-based platform designed to ease data collection for Omani public health programs. In addition, it helps in reporting different medical conditions and incidents and providing decision-makers with evidence-based results.18
Out of 426 TB patients in Muscat from 2017–2020, 115 TB-DM patients were included in the study. The inclusion criteria were all confirmed TB cases (either pulmonary TB or extrapulmonary TB), diagnosed and registered in Al Shifa 3+ records or registries. Patients were Omani and non-Omani citizens, > 13 years old, and residents of Muscat governorate with T2DM. The exclusion criteria included all confirmed TB cases with age < 13 years old, having type 1 DM (T1DM), or non-Muscat governorate residents. Also, people whose diabetes condition is unknown or unreported were excluded. T1DM was excluded because T2DM is more common in Oman. Additionally, the etiology of T1DM is different from T2DM and that might conflict with the results of the study and the susceptibility of getting TB.
The study data included all confirmed cases of TB for Omani and non-Omani citizens with T2DM in Muscat. The data was collected from the Al-Shifa 3+ system and variables were extracted from the TB e-notification form.
In this study, DM was the outcome variable, and sociodemographic, clinical characteristics, and comorbidities were associated factors for DM among TB patients. The sociodemographic variables are age, sex, marital status, nationality, place of residence (province), and occupation. Clinical characteristics of TB patients included body mass index (BMI) in m2/kg, Bacillus Calmette–Guérin (BCG) scar, TB site, TB status, TB drug resistance, and treatment outcome. Comorbidities associated with TB include the presence of HIV, hypertension, heart disease, chronic obstructive pulmonary disease, asthma, and renal disease, in addition to smoking and drinking alcohol.
Excel, Epi Info 7, and SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) were used for data extraction and data analysis. Calculations of frequency, percentage, means and SD were used to generate descriptive statistics. To examine the association between DM and sociodemographic, clinical features, and comorbidity variables, the chi-square test was used with a significance level of p-value ≤ 0.05. Multivariate logistic regression was performed on the variables identified as being significant by chi-square analysis.
Ethical approval (MoH/CSR/21/24815) was obtained from the Oman National Bioethics Committee. The ethical committee waived informed consent due to retrospective data review. To ensure confidentiality, the data was collected anonymously and without personal identifiers.
Results
There were 432 registered TB cases in Muscat during the study period. In the final analysis, 426 patients were retained and six excluded (five < 13 years and one with T1DM). A total of 115 patients of TB with DM were eligible for the study. In addition, 311 TB without DM were included in the comparative analysis [Figure 1].
 Figure 1: Flow chart on step-by-step selection and final analysis of study patients.
Figure 1: Flow chart on step-by-step selection and final analysis of study patients.
In this study, the mean age of TB-DM patients was 50.7±12.5 years. Over one-third (35.7%) of patients were ≥ 55 years old, which was followed by the 45–54 years age group (32.2%). The majority (75.7%) of the study participants were males with TB-DM. Most TB-DM patients were married (92.2%), and 62.6% were non-Omani citizens. The most common nationalities were Indian (30.4%), Bangladeshi (14.8%), Filipino (7.0%), and Pakistani (5.2%). The majority (78.3%) of TB-DM patients were employed, while a small percentage were unemployed (15.7%) or retired (6.1%). The reported patients were more in A'Seeb (33.0%) and Bowsher (28.7%) [Table 1].
Table 1: Comparison of sociodemographic distribution and clinical characteristics of TB patients enrolled in the study.
|
Age group
|
|
|
|
|
72.78
|
< 0.001
|
|
14–24
|
0 (0.0)
|
37 (11.9)
|
0.00 (0.00)
|
0.999
|
|
|
|
25–34
|
10 (8.7)
|
108 (34.7)
|
Reference
|
|
|
|
|
35–44
|
27 (23.5)
|
83 (26.7)
|
1.30 (0.52–3.27)
|
0.570
|
|
|
|
45–54
|
37 (32.2)
|
44 (14.1)
|
9.08 (4.16–19.84)
|
< 0.001
|
|
|
|
≥ 55
|
41 (35.7)
|
39 (12.5)
|
11.35 (5.19–24.82)
|
< 0.001
|
|
|
|
Mean ± SD
|
50.7 ± 12.5
|
38.4 ± 14.4
|
|
|
|
|
|
Gender
|
|
|
|
|
12.51
|
< 0.001
|
|
Male
|
87 (75.7)
|
177 (56.9)
|
2.35 (1.45–3.81)
|
|
|
|
|
Female
|
28 (24.3)
|
134 (43.1)
|
Reference
|
-
|
|
|
|
Marital status
|
|
|
|
|
44.92
|
< 0.001
|
|
Married
|
106 (92.2)
|
195 (62.7)
|
13.18 (4.72–36.84)
|
0.001**
|
|
|
|
Single
|
4 (3.5)
|
97 (31.2)
|
Reference
|
-
|
|
|
|
Divorced
|
2 (1.7)
|
0 (0.0)
|
0.00 (0.00)
|
0.999
|
|
|
|
Unknown
|
3 (2.6)
|
19 (6.1)
|
3.83 (0.79–18.50)
|
0.070
|
|
|
|
Nationality
|
|
|
|
|
24.38
|
< 0.001
|
|
Omani
|
43 (37.4)
|
107 (34.4)
|
4.01 (1.61–9.99)
|
0.002**
|
|
|
|
Indian
|
35 (30.4)
|
57 (18.3)
|
6.14 (2.40–15.70)
|
< 0.001
|
|
|
|
Bangladeshi
|
17 (14.8)
|
24 (7.7)
|
7.08 (2.50–20.12)
|
< 0.001
|
|
|
|
Pakistani
|
6 (5.2)
|
21 (6.8)
|
2.85 (0.83–9.83)
|
0.055
|
|
|
|
Filipino
|
8 (7.0)
|
42 (13.5)
|
1.90 (0.615–5.89)
|
0.139
|
|
|
|
Others*
|
6 (5.2)
|
60 (19.3)
|
Reference
|
-
|
|
|
|
Occupation
|
|
|
|
|
10.19
|
0.017*
|
|
Student
|
0 (0.0)
|
9 (2.9)
|
0.00 (0.00)
|
0.999
|
|
|
|
Employed
|
90 (78.3)
|
265 (85.2)
|
2.30 (1.19–4.47)
|
0.011*
|
|
|
|
Unemployed
|
18 (15.7)
|
23 (7.4)
|
Reference
|
-
|
|
|
|
Retired
|
7 (6.1)
|
14(4.5)
|
1.57 (0.52–4.69)
|
0.422
|
|
|
|
Wilayat
|
|
|
|
|
6.93
|
0.226
|
|
Al Amerat
|
12 (10.5)
|
28 (9.0)
|
1.94 (0.49–7.62)
|
0.340
|
|
|
|
Bowsher
|
33 (28.7)
|
84 (27.0)
|
2.12(0.60–7.43)
|
0.240
|
|
|
|
Muscat
|
7 (6.1)
|
13 (4.2)
|
1.55(0.34–6.94)
|
0.57
|
|
|
|
Muttrah
|
20 (17.4)
|
40 (12.9)
|
1.67 (0.45–6.13)
|
0.442
|
|
|
|
A'Seeb
|
38 (33.0)
|
140 (45.0)
|
3.07 (0.88–10.6)
|
0.076
|
|
|
|
Quriyat
|
5 (5.3)
|
6 (1.9)
|
Reference
|
-
|
|
|
|
BMI, kg/m2
|
|
|
|
|
5.91
|
0.116
|
|
Underweight (< 18.5)
|
6 (5.2)
|
27 (8.7)
|
Reference
|
-
|
|
|
|
Normal (18.5–24.9)
|
44 (38.3)
|
144 (46.3)
|
0.72 (0.28–1.87)
|
0.510
|
|
|
|
Overweight (25.0–29.9)
|
11 (9.6)
|
60 (19.3)
|
1.2 (0.40–3.61)
|
0.730
|
|
|
|
Obese (≥ 30.0)
|
4 (3.5)
|
4 (1.3)
|
0.22 (0.04–1.15)
|
0.073
|
|
|
|
Mean ± SD
|
23.3 ± 4.6
|
22.8 ± 3.7
|
|
|
|
|
|
BCG scar
|
|
|
|
|
6.98
|
0.030
|
|
Present
|
27 (23.5)
|
108 (34.7)
|
Reference
|
-
|
|
|
|
Absent
|
49 (42.6)
|
95 (30.5)
|
2.06 (1.19–3.56)
|
0.008**
|
|
|
|
Unknown
|
39 (33.9)
|
108 (34.7)
|
1.44 (0.83–2.52)
|
0.196
|
|
|
|
TB site
|
|
|
|
|
3.20
|
0.073
|
|
Pulmonary TB
|
101 (87.8)
|
250 (80.4)
|
0.56 (0.30–1.05)
|
0.076
|
|
|
|
Extrapulmonary TB
|
14 (12.2)
|
61 (19.6)
|
Reference
|
-
|
|
|
|
TB status
|
|
|
|
|
0.19
|
0.665
|
|
Newly diagnosed
|
112 (97.4)
|
305 (98.1)
|
1.43 (0.34–5.91)
|
0.619
|
|
|
|
Relapsed TB
|
3 (2.6)
|
6 (1.9)
|
Reference
|
-
|
|
|
|
TB drug resistance
|
|
|
|
|
0.10
|
0.753
|
|
Yes
|
11 (9.6)
|
33 (10.6)
|
0.605 (0.26–1.39)
|
0.238
|
|
|
|
No
|
104 (90.4)
|
278 (89.4)
|
Reference
|
-
|
|
|
|
TB treatment outcome
|
|
|
|
|
33.83
|
< 0.001
|
|
Cured
|
50 (44.2)
|
78 (25.1)
|
3.02 (1.71–5.31)
|
< 0.001
|
|
|
|
Completed treatment
|
16 (14.2)
|
74 (23.8)
|
1.40 (0.66–3.01)
|
0.365
|
|
|
|
Defaulter
|
4 (3.5)
|
12 (3.9)
|
1.57 (0.47–5.29)
|
0.467
|
|
|
|
Not Evaluated
|
9 (8.0)
|
19 (6.1)
|
2.23 (0.90–5.52)
|
0.077
|
|
|
|
Died
|
10 (8.8)
|
15 (4.8)
|
3.14 (1.26–7.82)
|
0.011*
|
|
|
TB: tuberculosis; DM: diabetes mellitus; OR: odds ratio; BMI: body mass index; BCG: Bacillus Calmette–Guérin.
*Others: Bahrani, Jordanian, Egyptian, Moroccan, Thai, Vietnamese, Bhutan. *p < 0.05; **p < 0.01.
The mean BMI of TB-DM patients was 23.3±4.6 kg/m2, with 38.3% being normal (p = 0.116). The absence of BCG scar in 49 (42.6%) TB-DM patients was statistically significant with a p-value < 0.05. In addition, most (87.8%) participants had pulmonary TB, while only 14 (12.2%) had extrapulmonary TB. The patients were distributed as follows: 112 newly diagnosed, and three relapsed TB. Eleven were drug-resistant TB. The TB outcomes were extremely significant (p < 0.001) with 44.2% cured (p < 0.001) TB-DM and 8.8% died (p < 0.011) [Table 1].
Thirty-six TB-DM patients had hypertension, six had cardiac diseases, 10 had renal diseases, and only one with HIV. In regards to smoking and alcohol drinking status among TB-DM patients, they were 36.5% and 30.4%, respectively [Table 2].
Table 2: Associated co-morbidities with TB patients enrolled in the study.
|
HIV status
|
|
|
|
1.50
|
0.221
|
|
Yes
|
1 (0.9)
|
9 (2.9)
|
0.294 (0.04–2.35)
|
|
|
|
No
|
114 (99.1)
|
302 (97.1)
|
Reference
|
|
|
|
Smoking status
|
|
|
|
19.92
|
< 0.001
|
|
Non-smoker
|
73 (63.5)
|
260 (83.6)
|
Reference
|
|
|
|
Smoker
|
42 (36.5)
|
51 (16.4)
|
2.93 (1.81–4.76)
|
|
|
|
Alcohol drinking status
|
|
|
|
5.63
|
0.015*
|
|
Non-alcoholic drinker
|
80 (69.6)
|
250 (80.4)
|
Reference
|
|
|
|
Alcoholic drinker
|
35 (30.4)
|
61 (19.6)
|
1.79 (1.10–2.91)
|
|
|
|
Hypertension
|
|
|
|
60.68
|
< 0.001
|
|
Yes
|
36 (31.3)
|
13 (4.2)
|
10.45 (5.29–20.64)
|
|
|
|
No
|
79 (68.7)
|
298 (95.8)
|
Reference
|
|
|
|
Heart disease
|
|
|
|
9.53
|
0.002**
|
|
Yes
|
6 (5.2)
|
2 (0.6)
|
8.50 (1.69–42.77)
|
|
|
|
No
|
109 (94.8)
|
309 (99.4)
|
Reference
|
|
|
|
Chronic obstructive pulmonary disease/asthma
|
|
2.25
|
0.134
|
|
Yes
|
0 (0.0)
|
6 (1.9)
|
Reference
|
|
|
|
No
|
115 (100)
|
305 (98.1)
|
0.00 (0.00)
|
|
|
|
Renal disease
|
|
|
|
10.63
|
< 0.001
|
|
Yes
|
10 (8.7)
|
6 (1.9)
|
4.84 (1.71–13.64)
|
|
|
TB: tuberculosis; DM: diabetes mellitus; OR: odds ratio.*p < 0.05; **p < 0.01.
From 2017 to 2020, the overall prevalence of T2DM among TB patients in Muscat was 27.0% (115/426) [Figure 1].
Male TB-DM (75.7%) patients were more than females (24.3%). Married patients made up the majority (92.2%) of patients than others (7.8%). The prevalence increased with age, from 8.7% in the 25–34 age group to 35.7% in the ≥ 55 year age group. The prevalence of TB-DM cases in Muscat increased from 27.5% in 2017 to 30.8% in 2020 with disparity with nationality [Figure 2]. Non-Omani citizens prevalence increased gradually from 7.5% in 2017 to 25.6% in 2020. However, Omani TB-DM prevalence decreased from 20.0% in 2017 to 5.1% in 2020 [Figure 3].
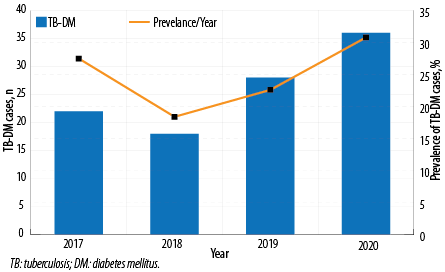 Figure 2: Prevalence of TB-DM cases in Muscat, 2017–2020.
Figure 2: Prevalence of TB-DM cases in Muscat, 2017–2020.
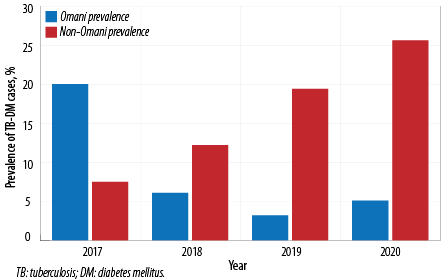 Figure 3: Prevalence of DM-TB among Omani and non-Omani in Muscat, 2017–2020.
Figure 3: Prevalence of DM-TB among Omani and non-Omani in Muscat, 2017–2020.
The data reveal that the prevalence of TB-DM in A'Seeb was high in 2017 and 2018 (7.7% and 9.2%, respectively). However, the prevalence was higher in Bowsher, with 9.8% in 2019 and 9.5% in 2020 [Table 3]. Over the four-year period, A'Seeb had the greatest number of non-Omani cases, except for 2019, when Bowsher had the greater number of cases (11 cases in comparison to nine cases in A'Seeb). Prevalence was distributed among the other wilayats. Quriyat and Muscat showed the lowest prevalence of TB-DM, whilst it was moderate in Al Amerat and Muttrah over the same period [Table 3].
Table 3: Prevalence of TB-DM cases according to the wilayat in Muscat.
|
Al Amerat
|
Omani
|
3
|
4.4
|
0
|
1.1
|
2
|
2.5
|
2
|
1.7
|
|
Non-Omani
|
1
|
|
1
|
|
1
|
|
0
|
|
|
Bowsher
|
Omani
|
1
|
4.4
|
2
|
4.1
|
1
|
9.8
|
2
|
9.5
|
|
Non-Omani
|
3
|
|
2
|
|
11
|
|
9
|
|
|
Muscat
|
Omani
|
2
|
3.3
|
0
|
0.0
|
0
|
1.6
|
0
|
1.7
|
|
Non-Omani
|
1
|
|
0
|
|
2
|
|
2
|
|
|
Muttrah
|
Omani
|
4
|
4.4
|
0
|
3.1
|
0
|
0.8
|
0
|
8.6
|
|
Non-Omani
|
0
|
|
3
|
|
1
|
|
10
|
|
|
A'Seeb
|
Omani
|
6
|
7.7
|
4
|
9.2
|
0
|
8.2
|
1
|
8.6
|
|
Non-Omani
|
1
|
|
5
|
|
9
|
|
9
|
|
|
Quriyat
|
Omani
|
2
|
2.2
|
0
|
1.1
|
1
|
0.8
|
1
|
0.9
|
|
Non-Omani
|
0
|
|
1
|
|
0
|
|
0
|
|
TB: tuberculosis; DM: diabetes mellitus.
Factors that were significantly associated with the occurrence of DM in TB patients include patients aged 40–49 years (OR = 9.08, 95% CI: 4.16–19.84) rising for ≥ 55 years (OR = 11.35, 95% CI: 5.19–24.82), male (OR = 2.35, 95% CI: 1.45–3.81), married (OR = 13.18, 95% CI: 4.72–36.84), employed (OR = 2.30, 95% CI: 1.19–4.47), and Bangladeshi (OR = 7.08, 95% CI: 2.50–20.12) or Indian (OR = 6.14, 95% CI: 2.40–15.70). The absence of BCG scar was also associated with DM in TB patients (OR = 2.06, 95% CI: 1.19–3.56). In addition, death (OR = 7.08, 95% CI: 1.26–7.82) and successful TB treatment (OR = 3.02, 95% CI: 1.71–5.31) were strongly associated with TB-DM (OR = 3.14 and 3.02, respectively). Other factors that were associated with TB-DM include hypertension (OR = 10.45, 95% CI: 5.29–20.64), heart disease (OR = 8.50, 95% CI: 1.69–42.77), renal disease (OR = 4.84, 95% CI: 1.71–13.64), smoking (OR = 2.93, 95% CI: 1.81–4.76), and drinking alcohol (OR = 1.79, 95% CI: 1.10–2.91)
Among all significant variables in univariate analysis, only age and hypertension made a statistically significant contribution to DM among TB patients, with adjusted odds ratios of 2.30 (95% CI: 1.72–3.06, p < 0.001) and 5.21 (95% CI: 2.28–11.87, p < 0.001), respectively [Table 4].
Table 4: Multiple logistic regression of associated factors for DM among TB cases in Muscat.
|
Age
|
0.833
|
2.30 (1.72–3.06)
|
< 0.001
|
|
Gender
|
-1.701
|
0.18 (0.08–0 .40)
|
< 0.001
|
|
Marital status
|
-0.946
|
0.38 (0.20–0.75)
|
0.005**
|
|
Nationality
|
-0.090
|
0.91 (0.76–1.09)
|
0.318
|
|
Occupation
|
-0.472
|
0.62 (0.42–0.91)
|
0.015*
|
|
BCG scar
|
-0.203
|
0.81 (0.62–1.06)
|
0.130
|
|
Outcome
|
0.079
|
1.08 (0.93–1.24)
|
0.276
|
|
Hypertension
|
1.651
|
5.21 (2.28–11.87)
|
< 0.001
|
|
Heart diseases
|
-0.955
|
0.38 (0.06–2.40)
|
0.307
|
|
Renal diseases
|
0.131
|
1.14 (0.30–4.20)
|
0.844
|
|
Smoking status
|
0.414
|
1.51 (0.96–2.36)
|
0.070
|
DM: diabetes mellitus; TB: tuberculosis; BCG: Bacillus Calmette–Guérin.
*p < 0.05; **p < 0.01.
Discussion
This is the first study in Oman to estimate the prevalence of DM in TB patients. It aims to determine the prevalence of T2DM and its associated factors among TB patients in Muscat, which will provide valuable information for comorbidity interventions. The overall prevalence of T2DM among TB was 27.0%, which is higher than what was reported in a similar study in Kerala (22.6%) in 2017 and Eritrea (9.88%) in 2016–2019, and less than what was reported in Vietnam (29%) in 2016 and Puducherry (29%) in 2014.8,9,13,19 Therefore, despite a decline in the prevalence of TB in Oman, the prevalence of diabetes continues to rise. Most TB-DM patients were non-Omanis. However, this can be accounted for 60.7% of Muscat’s total population being made up of non-Omanis.16 The increase in the prevalence of comorbidities among expatriates from 2017 (7.5%) to 2020 (25.6%) could merely reflect increases in the expatriate population. Also, most of the expatriates come from TB-endemic countries (India, Bangladesh, and Pakistan), have low socioeconomic income and lack medical insurance to access the health institutions for proper treatment and follow-up. In addition, the prevalence of these comorbidities rose from 27.5% in 2017 to 30.8% in 2020. The high prevalence of DM can also be attributed to TB, as DM can weaken the immune system and increase patients’ vulnerability to TB.20
Similar studies conducted in Vietnam in 2016 (13.7%), Bangladesh from 2013–2014 (12.8%), Denmark from 2009–2014 (5%), Ethiopia from 2013–2014 (11.5%), and India in 2012 (25.3%) revealed variations in the prevalence of DM among TB patients, which may be explained by sociodemographic characteristics of each population. The prevalence in Muscat (27.0%) is greater than all populations in the previously mentioned studies.9–12 Nevertheless, the prevalence of DM among TB patients in this study is lower than the reported percentages in related TB studies in some Middle Eastern countries, which share socioeconomic similarities with Oman such as Kuwait (29.8–35%), Iran (4.2–30%), and Iraq (41.1%).5 The alarming rise of DM among TB patients threatens TB control and treatment, and points to the importance of implementing collaborative comorbidity programs. For example, bidirectional screening programs of both diseases among Omani and non-Omani citizens.
In this study, the prevalence of DM among TB patients aged > 45 years increased significantly. This matches the findings of studies conducted in Vietnam (40–65 years), Bangladesh (45–59 years), Denmark (> 40 years), and Ethiopia (41–64 years).9–12 It has been established that the prevalence of DM and TB increases with age.21 This is due to a decline in immune function associated with aging, which increases the incidence of both diseases.22
The prevalence of DM among TB patients was highly significant in married, employed, and non-Omani males. The prevalence of DM in TB patients in males was double that found in females.9,10,19,23 Possible reasons could be that the frequency of smoking and drinking alcohol is higher among men, increasing their risks of developing both DM and TB.24 This study found a significant association between TB-DM in smoking and alcohol-drinking patients. TB patients who smoked were almost three times more likely to develop DM than non-smoking TB patients, and alcohol drinkers were 1.7 times more vulnerable to DM than TB patients who did not drink alcohol. These findings match research findings for Denmark and urban Puducherry.11,13
In our study, the prevalence of DM was seven times higher among Bangladeshi TB patients and six times higher among Indian TB patients compared to other nationalities. DM and TB are significantly associated in these two nationalities. Most of expatriate patients are typically of working age and reluctant to seek medical care because they lack medical insurance and fear being sent back home. The prevalence of TB in Bangladesh (404 per 100 000 population) and India (188 404 per 100 000 population) is high, while in Oman, the rate is low.25 Fortunately, there is existed policy for all expatriates to do medical investigations once in two years (visa medical), which includes rolling out active TB disease.26
According to the finding of this study, the majority of TB-DM patients reside in A'Seeb and Bowsher, which is consistent with expectations as these wilayats are the most densely populated in Muscat. Additionally, they are industrial areas that attract many residents and workers. Moreover, Indian and Bangladeshi workers constitute the majority of Asian nationalities seeking employment in Oman.27
Several clinical characteristics were observed in the current study of TB-DM patients. One of them is the absence of a BCG scar, which indicates either that the patient is not vaccinated against TB or a scar did not form after vaccination. This study found that the absence of BCG scar is highly associated with TB-DM patients. DM patients with TB are twice as likely to not have a BCG scar as those who do have a scar. However, it is important to note that the absence of a BCG scar does not indicate the absence of the vaccine’s effect; indeed, there are little relevant data on this issue.28–30
Also, this study found no association between BMI and DM in TB patients. Similar results were reported by an Indian medical journal.31 However, some data were missing from the patient’s records regarding the weights and heights that could affect these results. In addition, there was no relation between TB status (new or relapse), TB type (pulmonary or extrapulmonary), drug resistance, and comorbidity (TB-DM).
The current study found the cured and death outcome of TB treatment was highly significant. As the presence of DM with TB infection worsens, the prognosis deteriorates and treatment of TB is less effective, a death outcome is expected.32,33 Most of the newcomers to Oman with TB are treated and repatriated. Then, after they return to their home countries, the status of their condition is unknown.
The presence of other comorbidities may worsen the prognosis in TB-DM patients. Even though high blood pressure often occurs alongside DM, this study identifies that TB-DM patients are 10 times more likely to have hypertension than TB patients who do not have DM. DM damages the arteries, increasing the risk of developing atherosclerosis, which typically results in hypertension.34 In addition, the current study found that TB-DM patients are 8.5 times more likely to develop heart disease than non-DM TB patients do. Over time, high blood glucose can damage the vessels and nerves that regulate the heart’s function. Other significant risk factors for people with TB-DM are hyperlipidemia, smoking, and excessive alcohol consumption, as these increase the risk of cardiovascular diseases.35 The study also found that renal diseases were 4.5 times higher in TB-DM patients than in TB patients without DM. DM is associated with renal dysfunction because its direct effect on blood vessels causes microvascular complications such as nephropathy.36–39
Among all significant factors associated with DM in TB, being > 45 years or the presence of hypertension are the main predictors. Indeed, DM typically becomes more common with advancing age due to the aging process’s effect on the immune system. Moreover, hypertension is becoming increasingly prevalent with DM as its leading complication. These findings are consistent with the findings of a meta-analysis that old age and hypertension are risk factors for DM among TB patients.40
The primary strength of this study is that the results of glycated hemoglobin A1c and fasting blood sugar for DM diagnosis obtained in TB clinics make the diagnosis more reliable. In Oman, TB treatment is free of charge and all patients are treated regardless of their residence status. We can thus assume that no patients opted out of treatment due to financial reasons. Regarding the study limitation, due to the nature of the study, data were collected retrospectively from TB patients’ records and registries. Therefore, some data were missing, and errors may have occurred in transferring paper-based data to computer-based data. Furthermore, since the sample was from Muscat governorate only, generalizing the results to other regions of Oman, is not adequate. Thus, additional studies in other regions may be required. Also, since a significant proportion of TB patients were expatriates, who were treated and repatriated, they represent incomplete data points because it is not known what treatment was continued in their countries or the outcomes. This may underestimate the outcomes of TB treatment and the final progress of cases with comorbidities. Lastly, this study does not study the temporality.
Conclusion
This study finds the prevalence of diabetes among TB patients in Muscat to be significant; there is a concomitant high prevalence of raised blood sugar in the same area. It is more prevalent in non-Omanis than Omanis. This high prevalence may challenge the control of TB and DM in Muscat. Age and hypertension were found to be the most reliable predictors of DM among TB patients. Bidirectional TB-DM screening needs more attention. According to the World Health Organization Collaborative Framework for Care and Control of Tuberculosis and Diabetes, it is essential that all TB patients be screened for DM. Since Oman has a low TB incidence, latent TB screening among DM patients is important for early diagnosis. Furthermore, special attention is required for associated factors when managing these comorbidities. As the duration of TB-DM treatment is lengthy, future research is needed to identify factors that affect the duration and outcome.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes. Geneva: World Health Organization; 2015.
- 2. Ottmani SE, Murray MB, Jeon CY, Baker MA, Kapur A, Lönnroth K, et al. Consultation meeting on tuberculosis and diabetes mellitus: meeting summary and recommendations [Meeting report]. The International Journal of Tuberculosis and Lung Disease 2010 Dec 1;14(12):1513-1517.
- 3. Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C, et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn 2007 Sep;3(3):228-245.
- 4. Zheng C, Hu M, Gao F. Diabetes and pulmonary tuberculosis: a global overview with special focus on the situation in Asian countries with high TB-DM burden. Global Health Action 2017 Jan;10(1):1264702.
- 5. Alkabab YM, Al-Abdely HM, Heysell SK. Diabetes-related tuberculosis in the Middle East: an urgent need for regional research. International Journal of Infectious Diseases. Elsevier B.V 2015:64-70.
- 6. Ministry of Health. Annual health report 2020. [cited 2022 May 31]. Available from: https://www.moh.gov.om/en/web/statistics/-/-2020.
- 7. Al-Mawali A, Jayapal SK, Morsi M, Al-Shekaili W, Pinto AD, Al-Kharusi H, et al. Prevalence of risk factors of non-communicable diseases in the Sultanate of Oman: STEPS survey 2017. PLoS One 2021 Oct;16(10):e0259239.
- 8. Araia ZZ, Mesfin AB, Mebrahtu AH, Tewelde AG, Osman R, Tuumzghi HA. Diabetes mellitus and its associated factors in tuberculosis patients in Maekel Region, Eritrea: analytical cross-sectional study. Diabetes Metab Syndr Obes 2021 Feb;14:515-523.
- 9. Hoa NB, Phuc PD, Hien NT, Hoa VQ, Thuong PH, Anh PT, et al. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in Hanoi, Vietnam. BMC Infect Dis 2018 Nov;18(1):603.
- 10. Sarker M, Barua M, Guerra F, Saha A, Aftab A, Latif AM, et al. Double trouble: prevalence and factors associated with tuberculosis and diabetes comorbidity in Bangladesh. PloS One 2016 Oct 31;11(10):e0165396.
- 11. Huber FG, Kristensen KL, Holden IK, Andersen PH, Bakir B, Jørgensen A, et al. The prevalence of diabetes among tuberculosis patients in Denmark. BMC Infect Dis 2022 Jan;22(1):64.
- 12. Gedfew M. Predictors of extrapulmonary tuberculosis among diabetic patients at Debre Markos compressive specialized hospital, Ethiopia, 2021: a retrospective cohort study. J Clin Tuberc Other Mycobact Dis 2021 Oct;25:100280.
- 13. Raghuraman S, Vasudevan KP, Govindarajan S, Chinnakali P, Panigrahi KC. Prevalence of diabetes mellitus among tuberculosis patients in urban Puducherry. N Am J Med Sci 2014 Jan;6(1):30-34.
- 14. Shaikh MA, Singla R, Khan NB, Sharif NS, Saigh MO. Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Medical Journal 2003 Mar 1;24(3):278-281.
- 15. El-Hini S, Farghaly E, Osmanet A. Relation between type 2 diabetes mellitus and pulmonary tuberculosis: clinical implication and insulin sensitivity] El-minia. Med Bull (NY) 2007;18:157-170.
- 16. eCensus Portal. Interactive map population, housing, and establishments. 2020 [cited 2022 May 31]. Available from: https://portal.ecensus.gov.om/ecen-portal/?lang=en.
- 17. WHO. | Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. WHO. 2013 [cited 2022 May 31]. Available from: https://apps.who.int/iris/handle/10665/43588.
- 18. Introduction - Ministry of Health. Directorate General of Information Technology. [cited 2022 May 31]. Available from: https://www.moh.gov.om/en/web/directorate-general-of-information-technology.
- 19. Jacob S, George L, Joy A, Mathew M, Vijayakumar K, Kumar A, et al. Prevalence of diabetes mellitus and HIV/AIDS among tuberculosis patients in Kerala. J Family Med Prim Care 2020;9(12):6209-6212.
- 20. Restrepo BI. Diabetes and tuberculosis. Microbiol Spectr 2016;4(6):10.
- 21. Amare H, Gelaw A, Anagaw B, Gelaw B. Smear positive pulmonary tuberculosis among diabetic patients at the Dessie referral hospital, Northeast Ethiopia. Infectious Diseases of Poverty 2013 Mar;2(1):1-8.
- 22. García-Elorriaga G, Dis GD. Type 2 diabetes mellitus as a risk factor for tuberculosis. Mycobacterial Diseases 2014 [cited 2022 Nov 23];4:144. Available from: https://www.longdom.org/open-access-pdfs/type-diabetes-mellitus-as-a-risk-factor-for-tuberculosis-2161-1068.1000144.pdf.
- 23. Nagar V, Gour D, Pal DK, Singh AR, Joshi A, Dave L. A study on prevalence of diabetes and associated risk factors among diagnosed tuberculosis patients registered under Revised National Tuberculosis Control Programme in Bhopal District. J Family Med Prim Care 2018;7(1):130-136.
- 24. Silva DR, Muñoz-Torrico M, Duarte R, Galvão T, Bonini EH, Arbex FF, et al. Risk factors for tuberculosis: diabetes, smoking, alcohol use, and the use of other drugs. J Bras Pneumol 2018 Apr;44(2):145-152.
- 25. World Health Organization. Tuberculosis. 2022 [cited 2022 Jun 11]. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- 26. Khoja TA. Rules and regulations for medical examination of expatriates recruited for work in the Arab States of the Gulf Cooperation Council. Riyadh: Excutive Board of the Health Ministers’ Council 2010:65.
- 27. National Center for Statistics and Information. 2019 Population Statistics. 2019 [cited 2022 Jun 11]. Available from: https://www.ncsi.gov.om/Elibrary/LibraryContentDoc/bar_population 2019_dbf9c071-c68e-4907-ba03-6ad6aaf6ffdb.pdf.
- 28. Jason J, Archibald LK, Nwanyanwu OC, Kazembe PN, Chatt JA, Norton E, et al. Clinical and immune impact of Mycobacterium bovis BCG vaccination scarring. Infect Immun 2002 Nov;70(11):6188-6195.
- 29. Joshi L, Ponnana M, Sivangala R, Chelluri LK, Nallari P, Penmetsa S, et al. Evaluation of TNF-α, IL-10 and IL-6 cytokine production and their correlation with genotype variants amongst tuberculosis patients and their household contacts. PLoS One 2015 Sep;10(9):e0137727.
- 30. Ponnana M, Pydi S, Gaddam S. Enumeration of lymphocyte subsets during follow-up in the pulmonary tuberculosis patients with co morbid diabetes mellitus. Clin Chim Acta 2020 Nov;510:566-572.
- 31. Jain MK, Baghel PK, Agrawal R. Study of impaired glucose tolerance in pulmonary tuberculosis. Indian Journal of Community Medicine 2006 Jul 1;31(3):137.
- 32. Viswanathan AA, Gawde NC. Effect of type II diabetes mellitus on treatment outcomes of tuberculosis. Lung India 2014 Jul;31(3):244-248.
- 33. Ahmad SR, Yaacob NA, Jaeb MZ, Hussin Z, Wan Mohammad WM. Effect of diabetes mellitus on tuberculosis treatment outcomes among tuberculosis patients in Kelantan, Malaysia. Iran J Public Health 2020 Aug;49(8):1485-1493.
- 34. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol 2018 May;34(5):575-584.
- 35. Centers for Disease Control and Prevention. Diabetes and your heart | CDC. [cited 2022 May 31]. Available from: https://www.cdc.gov/diabetes/library/features/diabetes-and-heart.html.
- 36. Sowjanya Naha MD, Gardner MJ, Darshan Khangura MD, Kurukulasuriya LR, Sowers JR. Hypertension in diabetes. Interventions;2:5.
- 37. Omar N, Wong J, Thu K, Alikhan MF, Chaw L. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in Brunei Darussalam: a 6-year retrospective cohort study. Int J Infect Dis 2021 Apr;105:267-273.
- 38. Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PLoS One 2016 Jan 1;11(1):e0147621.
- 39. Hussein MM, Mooij JM, Roujouleh H. Tuberculosis and chronic renal disease. Semin Dial 2003;16(1):38-44.
- 40. Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS One 2017 Apr;12(4):e0175925.