Anti-Müllerian hormone (AMH), also known as Müllerian-inhibiting substance, is produced by Sertoli cells of the testis in males and by ovarian granulosa cells in females. In the absence of AMH, the Müllerian ducts and structures develop into the female reproductive tract. Hence, AMH is essential for the involution of the Müllerian ducts (the anlagen of the internal female genitalia in the male fetus) which would otherwise differentiate into the uterus and fallopian tubes.1 In males, AMH induces regression of the Müllerian duct, allowing Wolffian ducts to develop into the male reproductive tract under the influence of testosterone; this process occurs as early as 8 weeks of intrauterine life.2,3 AMH is continuously produced by the testes until puberty, and then levels decrease slowly to post-puberty values.4 In the absence of AMH, the embryo develops into a female, allowing the Müllerian duct to differentiate into the upper vagina, uterus, and oviduct. Thus, AMH is used for the differential diagnosis of disorders of sex development.5
During reproductive ages in women, the circulating levels of AMH reflect the number of remaining primordial follicles. The role of AMH in ovarian physiology is important in many fields related to women’s health, such as ovarian reserve and ovarian dysfunction testing. Areas of reproductive medicine where ovarian reserve testing by AMH include the diagnosis and management of female infertility including in vitro fertilization (IVF) and prediction of pregnancy, and diagnosis and prediction of menopause. The AMH test also contributes to the diagnosis and management of women with polycystic ovarian syndrome (PCOS), gynecological cancer, particularly its help to identify which chemotherapeutic agents are toxic to the ovaries and other disorders.5
Methods
A total of 319 healthy women were recruited for the study after receiving study information and consent forms. The participants were in their reproductive to menopause ages (20–50 years). The exclusion criteria included women with menstrual irregularities, PCOS, high luteinizing hormone (LH), and follicle-stimulating hormone (FSH) values corresponding to the menstrual cycle, infertility (including those on treatment for infertility or on follow-up for the same), history of gynecological operations such as hysterectomy and oophorectomy, or chronic diseases such as diabetes, hypertension, cardiovascular disease, thyroid disorder, cancer, or obesity (body mass index (BMI) > 30 kg/m2). Women who were using oral estrogen/progesterone contraceptive pills were also excluded. The study protocol was approved by the Medical Ethics and Scientific Research Committee, Royal Hospital, Muscat (Ref. MESRC#72/2016).
To assess ovulation, the participants were requested, when possible, to present on their mid-luteal day (day 21 of the current menstrual cycle or seven days before the subsequent cycle), and ovulation was confirmed by measurement of serum progesterone. Where it was difficult to come on such day, a random sample was taken regardless of the day of the cycle. The women were categorized based on their serum progesterone levels.
Blood specimens were collected using serum-separating gel-containing tubes. Serum samples were then aliquoted for measurement of FSH, LH, progesterone, and AMH. Samples not analyzed on the same day were stored at -20 °C according to the manufacturer’s protocol to ensure sample stability.
Serum AMH levels were analyzed using AMH Elecsys assay on electro-chemiluminescence immunoassay analyzer (Cobas-e601, Roche). Two quality control specimens were analyzed for each run. The reportable range of the assay was > 164 pmol/L, lower limit of quantification was 0.214 pmol/L, and lower limit of detection was 0.07 pmol/L. Measurements of FSH, LH, and progesterone were performed using the equipment Architect i2000 Immunology Analyzer (Abbott, USA).
Statistical analysis was performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics, Version 24.0. Armonk, NY: IBM Corp.) and R version 3.3.3 (The R Project for Statistical Computing). Kruskal-Wallis test was used to compare skewed data in different age groups, and analysis of variance (ANOVA) was used to compare normalized data in different age groups.
We followed the Clinical and Laboratory Standards Institute guideline to establish the reference range for AMH at different age groups. We first tested the normal distribution of variables using the Shapiro-Wilk test, density, and quantile-quantile plots. To normalize AMH values, we applied various data transformations: reciprocal, logarithmic (10 and natural base), square root, square, and cubic. For age-dependent reference age estimation, we first identified the best-fit regression model between the age and normalized serum AMH levels using power, exponential, quadratic, cubic, and linear models.
Results
The subjects comprised N = 319 women aged 20–50 years. They were classified into six age groups: 20–25 years (n = 57), 26–30 years (n = 84), 31–35 years (n = 70), 36–40 years (n = 64), 41–45 years (n = 33), and 46–50 years (n = 11) (Table 1). We observed an age-dependent increase in BMI (p <0.001) and no difference was observed in HbA1c, LH, FSH, and progesterone.
Table 1: Clinical and laboratory characteristics of the study population (N = 319).
|
Number of participants
|
57
|
84
|
70
|
64
|
33
|
11
|
-
|
|
Age, years
|
23
|
28
|
33
|
38
|
43
|
46
|
-
|
|
BMI, kg/m2
|
20.98
(15.80–30.0)
|
24.00
(16.30–31.00)
|
24.45
(16.30–32.00)
|
27.00
(16.00–32.70)
|
27.50
(21.50–32.00)
|
26.00
(21.50–29.00)
|
< 0.001
|
|
HbA1c, %
|
4.6
(4.1–5.5)
|
5.1
(4.3–6.1)
|
5.3
(4.1–6.1)
|
4.8
(4.3–6.1)
|
5.1
(4.5–6.1)
|
5.4
(5.4.6.1)
|
0.071
|
|
LH, IU/L
|
4.30
(0.88–13.73)
|
4.30
(0.74–22.92)
|
3.58
(0.05–13.20)
|
3.39
(0.69–14.60)
|
4.24
(0.90–9.10)
|
3.40
(1.05–24.45)
|
0.335
|
|
FSH, IU/L
|
3.87
(1.10–8.93)
|
4.18
(1–10.41)
|
4.48
(0.20–11.55)
|
3.46
(1.13–10.75)
|
4.95
(1.78–17.11)
|
11.12
(1.82–22.70)
|
0.068
|
The above data give median values (ranges in parentheses). P-values were determined using Kruskal-Wallis test.
BMI: body mass index; HbA1c: glycated hemoglobin; LH: luteinising hormone; FSH: follicle-stimulating hormone.
The distribution of serum AMH values was left-skewed (p < 0.001) [Figure 1a]. Values were transformed using logarithmic (natural and base 10), square root, cubic, square, and reciprocal methods, [Figure 1b–e]. We observed normal distribution with square root transformation of serum AMH levels with a normal distribution density plot and fitted dots within the Q–Q plot line [Figure 1e] and Shapiro-Wilk p-value of 0.300. Subsequent analyses were performed using the square root values of serum AMH. Levels of AMH were significantly negatively correlated with age with Pearson correlation coefficient r = -0.474 (p < 0.001) [Figure 2a]. Serum levels of AMH were significantly different in different age groups (ANOVA p < 0.001) [Figure 2b].
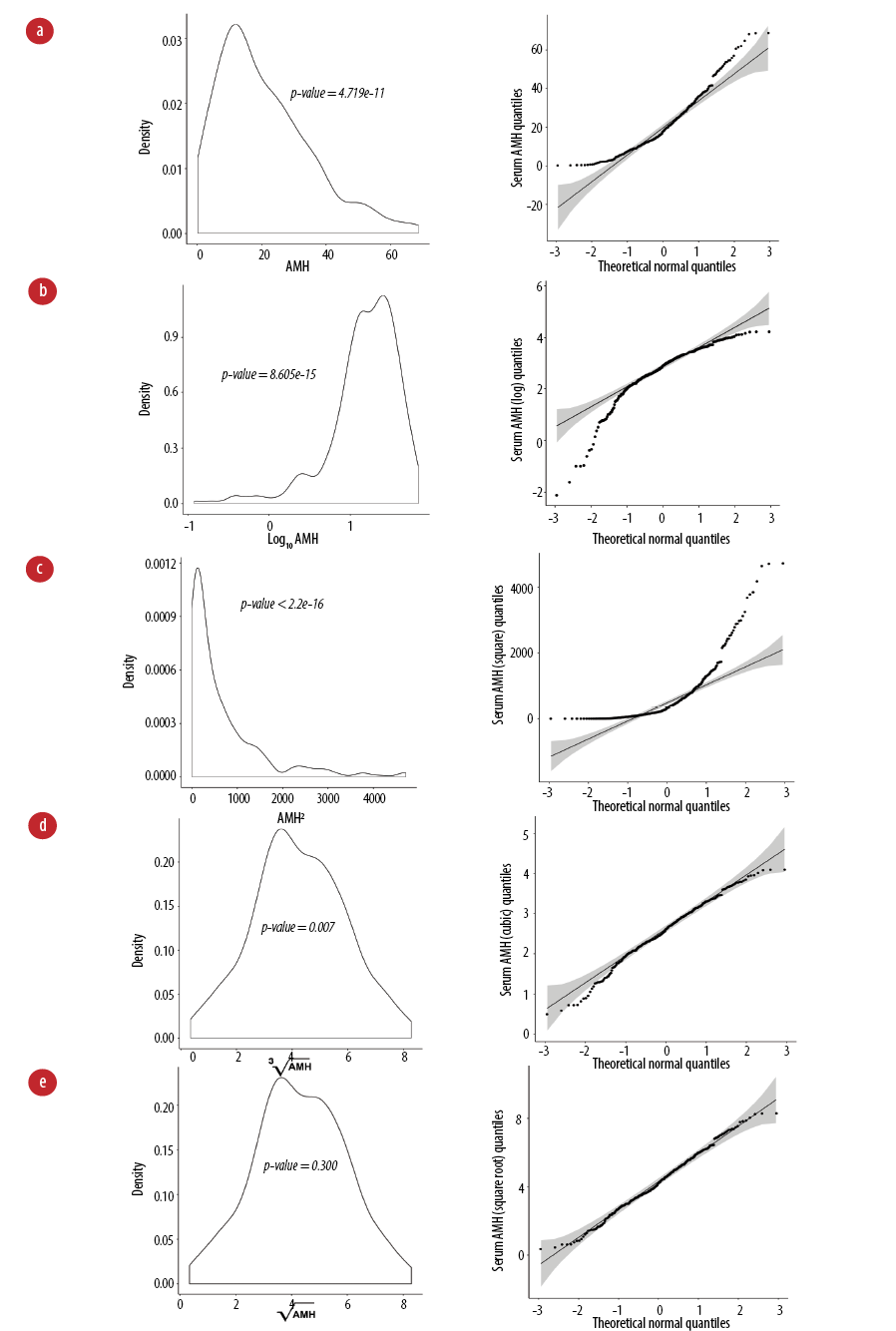 Figure 1: Transformation of serum anti-Müllerian hormone (AMH) (pmol/L) in the study population with age. (a) Kernel density plot (left) and quantile-quantile plot (right) are presented for non-transformed AMH values and (b) transformation using logarithmic base 10, (c) square, (d) cubic, and (e) square root. Shapiro-Wilk p-value > 0.05 indicates a normal distribution.
Figure 1: Transformation of serum anti-Müllerian hormone (AMH) (pmol/L) in the study population with age. (a) Kernel density plot (left) and quantile-quantile plot (right) are presented for non-transformed AMH values and (b) transformation using logarithmic base 10, (c) square, (d) cubic, and (e) square root. Shapiro-Wilk p-value > 0.05 indicates a normal distribution.
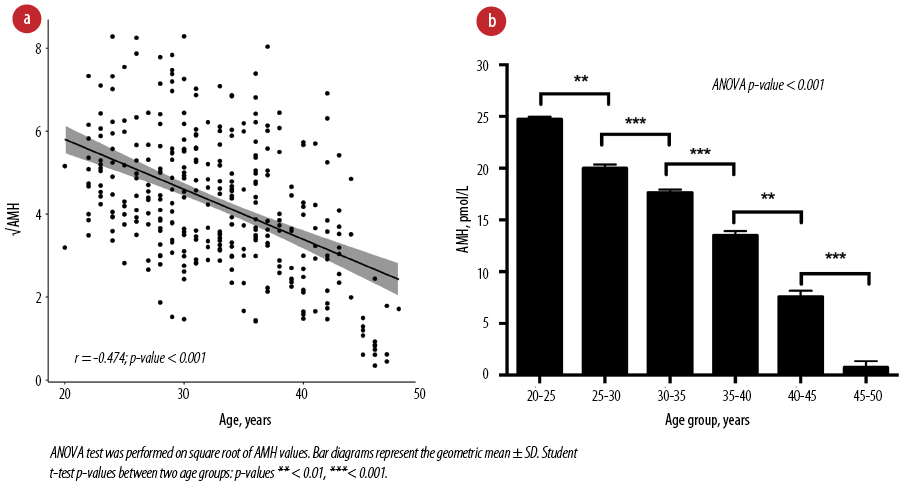 Figure 2: Correlation of serum anti-Müllerian hormone (AMH) with age. (a) Square root values of serum AMH levels were used for correlation. Pearson correlation test (r) was performed. (b) Serum AMH levels in different age groups in the study population.
Figure 2: Correlation of serum anti-Müllerian hormone (AMH) with age. (a) Square root values of serum AMH levels were used for correlation. Pearson correlation test (r) was performed. (b) Serum AMH levels in different age groups in the study population.
We implemented various models (linear, quadratic, cubic, exponential, and power) to study the goodness of fit between the correlation and the square root of AMH and age [Table 2]. The exponential model was the best-fit model with a highest R2 of 0.298 compared to other models. The exponential model √AMH = 479.02 × 0.91age was further used to build the nomogram for reference ranges of AMH per age in years.
Table 2: Predication models of age-dependent serum anti-Müllerian hormone (AMH) levels.
|
Power
|
√AMH = 260.6 × age-1.22
|
106.3
|
< 0.001
|
0.260
|
|
Exponential
|
√AMH = 479.02 × 0.91age
|
128.2
|
< 0.001
|
0.298
|
|
Quadratic
|
√AMH = -5.87age2 – 13.97age + 4.26
|
61.5
|
< 0.001
|
0.273
|
|
Cubic
|
√AMH = 2.83age3 – 5.87age2 – 13.97age + 4.26
|
43.4
|
< 0.001
|
0.286
|
Age: use in years. AMH was reported in pmol/L.
The square root of AMH values was log-transformed to generate an exponential model with age, followed by calculation of age-specific 0.50, 0.10, 0.25, 0.50, 0.75, 0.90, and 0.95 quintiles of log AMH. The quintile values obtained were returned geometric values by the anti-log return of square root. Then, the AMH-age nomogram was generated using geometric quintile values. This nomogram clearly demonstrates the negative relationship between AMH and age, thereby providing guidance on the relative value of an individual’s AMH given her age [Figure 3].
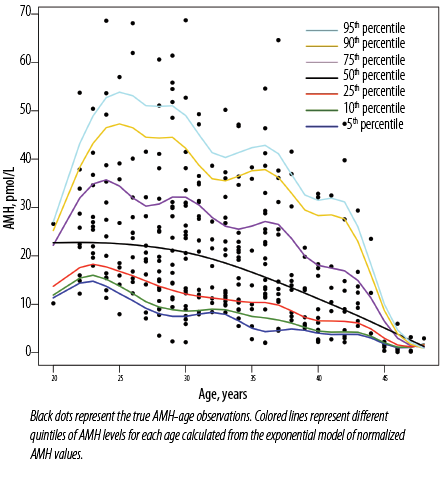 Figure 3: Anti-Müllerian hormone (AMH)-age nomogram (age-specific centiles).
Figure 3: Anti-Müllerian hormone (AMH)-age nomogram (age-specific centiles).
The reference ranges for AMH between the 2.5th and the 97.5th percentile for the six age groups are given in Table 3, and summarized in Table 4.
Table 3: Percentile of serum Anti-Müllerian hormone levels (pmol/L) in different age groups.
|
2.5th
|
10.63
|
3.74
|
5.49
|
2.15
|
0.92
|
0.14
|
|
5th
|
12.01
|
6.90
|
7.61
|
2.57
|
1.32
|
0.15
|
|
10th
|
14.54
|
8.59
|
8.40
|
4.66
|
1.75
|
0.20
|
|
25th
|
18.23
|
13.32
|
11.03
|
7.45
|
3.41
|
0.37
|
|
50th
|
26.61
|
20.89
|
19.92
|
13.71
|
9.24
|
0.68
|
|
75th
|
35.83
|
31.09
|
28.12
|
25.48
|
14.80
|
1.59
|
|
90th
|
45.71
|
51.43
|
37.06
|
37.13
|
28.99
|
3.20
|
|
95th
|
53.65
|
59.37
|
46.93
|
41.43
|
35.23
|
4.37
|
Table 4: Recommended reference ranges for anti-Müllerian hormone (AMH) according to age group in the study population.
|
20–25
|
10.63–55.64
|
|
26–30
|
3.74–61.88
|
|
31–35
|
5.49–47.56
|
|
36–40
|
2.15–48.91
|
|
41–45
|
0.92–41.26
|
Discussion
AMH in females is produced in the ovary by granulosa cells of antral follicles. AMH level is a clinically used marker to assess the ovarian reserve for the diagnostics of infertility, premature ovarian failure, and hypogonadotropic hypogonadism. Here, we report an age-specific reference range of serum AMH in healthy Omani women at various reproductive age groups till menopause.
We evaluated the hormonal states of all participating women by measuring serum AMH, FSH, LH, and progesterone. Women with progesterone < 20 nmol/L and ≥ 20 nmol/L had negligible differences in levels of AMH (4.3 ± 0.1 pmol/L and 4.0 ± 0.1 pmol/L, respectively). This finding supports the clinical practice of investigating a woman’s fertility status by measuring the serum AMH level of blood collected on any day of the menstrual cycle. This is in contrast to measuring the levels of FSH, LH, and progesterone, which vary with the phase of the cycle and status of ovulation.
Ovarian aging is related to the decline in the quantity and quality of the ovarian follicle pool that physiologically occurs with increasing age.6 This decline in follicle quality is due to the morphological changes that occur with ageing; metabolic factors that involve the mitochondria, smooth endoplasmic reticulum, and Golgi complex; and their effect on follicular growth given the increase in metabolic requirements.7 We observed a significant decline in the levels of AMH from the age of 20, with a more rapid decline after 40 [Figure 2b]. Van Disseldorp et al,8 reported AMH levels remaining stable between 8 and 25 years and declining after 30. Soto et al,9 demonstrated that AMH levels begin to decrease at 33 years of age. As per the American College of Obstetricians and Gynecologists Committee on gynecologic practice, female fecundity begins to decline significantly at approximately 32 years of age and decreases more rapidly after 37 years.10
Ethnic differences may be involved in some gynecological problems associated with hormonal levels. For example, Indian subcontinent and Asian women have higher rates of PCOS and insulin resistance compared with other ethnicities.11 Significant disparities in assisted reproductive technology study outcomes have been reported between ethnicities in outcomes such as pregnancy, live birth, growth restriction, and preterm birth.12 Another study found that healthy white women aged 25–45 years had lower AMH levels compared with African-American, Latin, and Chinese women.13 On the other hand, Olcha et al,14 evaluated the relationship between genetic ethnicity and ovarian reserve/response as evidenced by FSH, AMH, basal antral follicle count, and total oocyte yield in infertile women undergoing IVF at a single center. After controlling for age and BMI, genetic ethnicity had no impact on AMH, basal antral follicle count, and oocyte yield.14 Similarly, in another study on women attending fertility clinics observed no differences in AMH levels among different ethnic groups.15 Lack of ethnic differences in these two studies14,15 could be due to the involvement of women attending infertility clinics than healthy fertile women.13
Table 5 summarizes AMH data from the Omani Arab women in the current study compared with other populations reported in the literature. Overall, the median and ranges of AMH levels in Arab women appear to be slightly lower than in the age-matched women from Belgium, Brazil, France, Germany, Netherland, and Turkey using automated AMH Elecsys assay,17 Immunotech assay,19 AMH-GenII assay,20 and AMH-GenII assay.18 However, AMH levels of the Omani Arab women were slightly higher compared with those of Persian women for whom the AMH-GenII assay was used.16 This finding suggests that Middle Eastern populations tend to have an earlier menopause than European populations, which supports the use of AMH as an indicator of ovarian age at the time of menopause.
The differences in AMH levels between studies may be attributed to the differences in ethnicities, nationalities, lifestyle, environmental factors, and socioeconomic status.9 Additionally, age-related variables such as ovarian reserve, menopause, ovarian follicle numbers, AMH could be affected by genetic factors within the same ethnic groups. Candidate genes and single nucleotide polymorphisms (SNPs) are associated with ovarian follicle numbers in European and African ancestries.17 The SNP rs6543833 and several nominal variants nearby and within the myeloid-associated differentiation marker-like gene are associated with FSH levels in African American women.21 Additionally, significant numbers of potentially damaging variants in minichromosomal maintenance 8 and 9 (MCM8 and MCM9) have been shown in patients with primary ovarian insufficiency, and multiallelic associated variants in the DNA damage repair pathway and MCM8-MCM9 interacting genes might contribute to functional ovarian biology.
Additionally, the majority of AMH studies reported their data from women attending infertility or obstetrics and gynecology clinics with possible effects on many variables on AMH values. The variation in sample sizes and the different assay methodologies used in these studies might have additionally contributed to the variation in AMH levels. The majority of studies used the AMH gen II ELISA technique, and we used Elecsys Cobas (Roche; Germany), which correlates well with ELISA methods but typically provides 24–28% lower values.17 Additionally, some of these studies reported the reference range as 5th and 95th percentile ranges, whereas some reported the ranges as 2.5th and 97.5th percentile ranges, similar to this study.
The limitations of the study were: though we followed the standard guidelines in establishing the reference ranges, the cut-off values were not compared with control populations. Also, most of our subjects were from the northern urbanized region of Oman and may not be representative of the nation’s ethnically diverse population. We recommend a more detailed study with participants representing all major regions and ethnicities of Oman, and compared with control populations from the regions to effectively address these limitations.
This was the first study to determine the reference range of serum AMH in healthy Omani women with normal menstrual cycles during their reproductive to menopausal ages. The age-specific reference ranges we obtained are expected to aid in the interpretation of AMH values in Omani women while assessing their ovarian function and reserve in clinical practice.
The authors declared no conflicts of interest. No funding was received for this study.