Malignant otitis externa (MOE) is an invasive infection of the external auditory canal (EAC) and base of the skull.1–3 It is termed malignant because it behaves and spreads like a malignancy.1 The infection predominantly affects patients with primary or secondary immunodeficiency, such as older people with diabetes (in 90% of cases), patients undergoing radio- or chemotherapy, those with HIV infection, and children with malnutrition.4 Multiple bacterial and fungal organisms have been identified as causes of MOE; however, Pseudomonas aeruginosa is the most common causative agent.2,4
Patients with MOE usually present with persistent otalgia, otorrhea, and hearing loss.5,6 The disease can be complicated by severe osteomyelitis of the temporal bone, palsy of the cranial VII–XII nerves (particularly the facial nerve), and involvement of the blood vessels and soft tissues. In addition, death can occur due to extensive osteomyelitis of the skull and disseminated septic thromboembolism of the brain.5,6 A diagnosis of MOE is made from a combination of clinical, laboratory, and radiological findings and nuclear imaging.2 Computed tomography (CT) and magnetic resonance imaging (MRI) scans are used to determine the anatomical extent of the disease. Radioisotope imaging using technetium-99/gallium-67 is a sensitive but non-specific test that is usually used to monitor treatment response as it detects actively dividing cells.4
Treatment of MOE requires the prolonged use of local or systemic antibiotics—with ear swabs guiding the choice of antimicrobial therapy—as well as diabetes control measures and repeated debridement of the necrotic tissue; in some cases, aggressive surgical management may be necessary.1,5 Hyperbaric oxygen therapy (HBOT) involves the intermittent breathing of 100% oxygen while under conditions in which the ambient pressure is greater than atmospheric pressure. Although HBOT has been considered an adjunctive therapy in the treatment of various infectious and inflammatory diseases, this modality is not yet indicated for use in MOE.3 However, there are reports in the literature supporting the beneficial effect of HBOT in the management of MOE cases that share similar characteristics to obstinate osteomyelitis and necrotizing inflammation.2–11
At the Said Bin Sultan Naval Base Polyclinic in Oman, HBOT has been offered as an outpatient-based service since 1979. Treatment consists of multiple sessions in which the patient is exposed to a pressure of 2.4 atmospheres absolute. A single daily session lasts for 90 minutes and involves three periods of pure oxygen breathing for 25 minutes at a time, interspersed by three five-minute intervals of air-breathing. The total number of treatment sessions required is individually prescribed according to each patient’s clinical manifestations and treatment response. The aim of this case series was to determine the effectiveness of HBOT as an adjuvant therapy for patients with MOE and to identify factors affecting treatment outcomes.
Case report
Between January 2014 and December 2019, 26 patients with MOE were referred for adjuvant HBOT to the Hyperbaric Medicine Unit of Said Bin Sultan Naval Base Polyclinic due to worsening of the disease following the failure of conventional treatment (i.e., antibiotic therapy with or without concurrent surgery). Six patients were excluded from the series as they did not complete the total number of recommended HBOT sessions for various reasons, including four patients who faced logistical and transportation difficulties and two patients who were lost to follow-up. Treatment outcome was classified into four categories: died of disease, died of other causes (DOC), cured with no evidence of disease, and alive but with refractory disease. The latter category was applied in cases in which there was incomplete resolution of the otalgia, otorrhea, or granulation tissue.
Ethical approval for this case series analysis was obtained from both the Forces Medical Services Medical Ethics Committee (#FMS-MEC 005/2020) and the Ministry of Health (#MoH/CSR/20/23816). Data were collected from the medical records system of the referring hospitals, as well as the in-house medical records system of the Said Bin Sultan Naval Base Polyclinic. Relevant information was collected using a data collection sheet and entered for statistical analysis. Overall, the mean age of the patients was 64.6 years and the mean follow-up period was 14.1 months. The patients underwent an average of 29.0±8.9 HBOT sessions (range: 13–51 sessions). All of the patients had diabetes.
Case one
A 63-year-old male with known uncontrolled diabetes and hypertension presented with a six-week history of persistent right ear pain and discharge. An ear swab culture showed no growth. Radiological imaging confirmed mastoiditis and temporal bone osteomyelitis. He received ciprofloxacin and ceftazidime for 47 days before HBOT and underwent a tympanomastoidectomy. The patient experienced a full recovery following 27 sessions of HBOT.
Case two
A 56-year-old female with known uncontrolled diabetes, hypertension, and chronic kidney disease (CKD) presented with persistent right ear pain, discharge, and right-sided facial nerve palsy of more than two weeks duration. An ear swab culture was negative. Mastoiditis was observed during radiological imaging. She was prescribed a prolonged course of ciprofloxacin and piperacillin/tazobactam for 17 days before undergoing 25 sessions of HBOT, after which the MOE resolved completely. However, the facial nerve palsy persisted.
Case three
A 64-year-old female with uncontrolled diabetes, hypertension, and CKD presented with a three-year history of persistent left ear pain and discharge. Investigations revealed a growth of P. aeruginosa from an ear swab culture. Mastoiditis was observed during both CT and Gallium-67 scans [Figure 1]. She received ciprofloxacin for 124 days and underwent 44 sessions of HBOT resulting in disease resolution [Figure 2]. The patient developed bilateral middle ear barotrauma as a side-effect of the HBOT, which was treated successfully with a nasal decongestant.
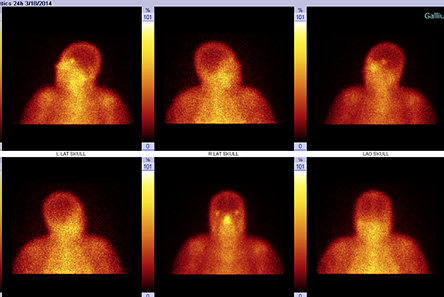 Figure 1: Case 3, before hyperbaric oxygen therapy treatment. Gallium-67 scintigraphy showing a focal area of increased tracer uptake in the left ear region including the left sphenoid bone with extension to the left mastoid process. Suggestive of active left otitis. (Date: 18/3/2014).
Figure 1: Case 3, before hyperbaric oxygen therapy treatment. Gallium-67 scintigraphy showing a focal area of increased tracer uptake in the left ear region including the left sphenoid bone with extension to the left mastoid process. Suggestive of active left otitis. (Date: 18/3/2014).
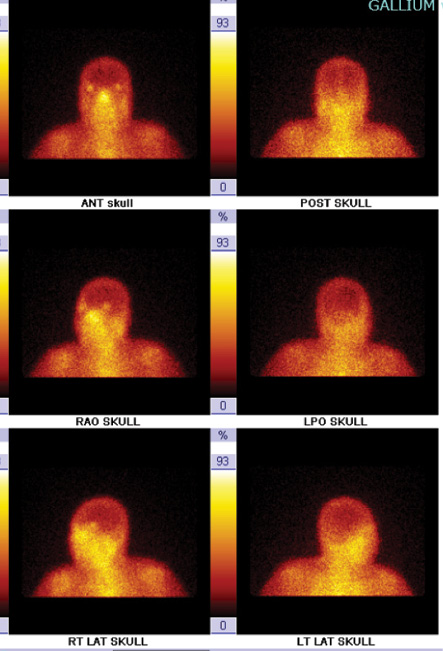 Figure 2: Case 3, after hyperbaric oxygen therapy treatment. Gallium-67 scintigraphy showed normal radiotracer uptake in the skull. (Date: 13/4/2015).
Figure 2: Case 3, after hyperbaric oxygen therapy treatment. Gallium-67 scintigraphy showed normal radiotracer uptake in the skull. (Date: 13/4/2015).
Case four
A 64-year-old male with known uncontrolled diabetes and hypertension presented with a three-month history of persistent left ear pain and discharge. P. aeruginosa was isolated from an ear swab culture and a CT scan confirmed mastoiditis and temporal bone osteomyelitis. Ceftazidime and piperacillin/tazobactam were prescribed for 84 days before referral to HBOT. The patient underwent 27 sessions of HBOT and was cured with no evidence of disease.
Case five
A 71-year-old male with known uncontrolled diabetes, hypertension, and CKD presented with a six-week history of persistent right ear pain and discharge. An ear swab culture was negative. Mastoiditis and temporal bone osteomyelitis were seen during radiological imaging. He received ceftazidime and ciprofloxacin for 45 days before referral to HBOT. A complete resolution of the MOE occurred after 25 sessions of HBOT.
Case six
A 39-year-old female with controlled diabetes presented with a three-week history of persistent left ear pain and discharge. An ear swab culture revealed the growth of P. aeruginosa. The disease was complicated by temporal bone osteomyelitis. The patient was prescribed a prolonged course of ciprofloxacin and piperacillin/tazobactam for 24 days before HBOT started. She underwent 30 sessions of HBOT, after which she had a full recovery.
Case seven
An 81-year-old male with uncontrolled diabetes presented with a six-month history of persistent left ear pain and discharge. An ear swab culture showed Klebsiella pneumoniae and Proteus mirabilis isolates. The MOE was complicated by mastoiditis, bone osteomyelitis of the skull base, jugular foramen syndrome, and cerebral vein and transverse sinus thrombosis. The patient received piperacillin/tazobactam and ciprofloxacin for 184 days before starting HBOT. He underwent 27 sessions of HBOT and recovered completely.
Case eight
A 62-year-old male with uncontrolled diabetes presented with persistent right ear pain, discharge, and impaired hearing of six weeks duration. An ear swab culture showed mixed growth of P. aeruginosa and Staphylococcus aureus. He received ceftazidime and meropenem for 43 days then was referred for HBOT. The patient underwent 17 sessions of HBOT and was subsequently cured of the disease.
Case nine
A 62-year-old male with known controlled diabetes and hypertension presented with a history of persistent right ear pain, discharge, and impaired hearing of more than two weeks duration. P. aeruginosa was isolated from an ear swab culture. An MRI showed osteomyelitis of the skull base. The patient received a prolonged course of piperacillin/tazobactam for 15 days before HBOT was initiated. After undergoing 27 sessions of HBOT, full resolution of the disease was observed.
Case 10
A 71-year-old female with controlled diabetes and hypertension presented with persistent left ear pain and discharge of more than two weeks duration. An ear swab culture was negative. However, a CT scan confirmed an extension of the infection to the infratemporal fossa and parapharyngeal space. The patient received ciprofloxacin and ceftazidime for 17 days then HBOT started. She made a full recovery after 25 HBOT sessions.
Case 11
An 85-year-old female with uncontrolled diabetes and hypertension presented with a six-month history of persistent left ear pain and discharge. An ear swab culture revealed multidrug-resistant P. aeruginosa. Both CT and gallium-67 scans confirmed temporal bone osteomyelitis. She received colistin for 168 days. Despite 51 sessions of HBOT, the disease persisted until the patient’s death as a result of a myocardial infarction after six months of treatment. Beforehand, the patient had developed bilateral middle ear barotrauma as a side-effect of HBOT, which was treated successfully with a nasal decongestant.
Case 12
A 65-year-old male with known uncontrolled diabetes and hypertension presented with four-month history of persistent left ear pain and discharge. An ear swab culture revealed P. aeruginosa isolates. Mastoiditis, temporal bone, and skull base osteomyelitis were observed during an MRI scan. The patient received a prolonged course of piperacillin/tazobactam for 134 days before undergoing 29 sessions of HBOT. He subsequently made a full recovery.
Case 13
A 60-year-old male with uncontrolled diabetes and hypertension presented with a four-month history of persistent right ear pain and discharge. Ear swab culture showed no growth. Both CT and gallium-67 imaging confirmed temporal bone osteomyelitis. The patient received piperacillin/tazobactam for 122 days and underwent a cortical mastoidectomy. He underwent 31 sessions of HBOT before making a full recovery.
Case 14
A 69-year-old male with controlled diabetes presented with persistent left ear pain and discharge of six weeks duration. An ear swab culture showed P. aeruginosa and Aspergillus flavus. Both MRI and technetium-99 imaging confirmed temporal bone osteomyelitis and involvement of the temporomandibular joint. The patient received ceftazidime, ciprofloxacin, and clotrimazole for 40 days. He underwent 16 sessions of HBOT, after which he was cured with no evidence of the disease.
Case 15
A 77-year-old female with known controlled diabetes and hypertension presented with a five-week history of persistent left ear pain, discharge, and left-sided facial nerve palsy. Ear swab culture was negative. However, a CT scan confirmed skull base, temporal bone osteomyelitis, internal jugular vein compression, and involvement of the retropharyngeal space. The patient received caspofungin and ceftazidime for 39 days. She underwent 33 sessions of HBOT with full resolution of both the MOE and the facial nerve palsy.
Case 16
A 59-year-old male with uncontrolled diabetes and hypertension presented with a history of persistent left ear pain, discharge, and left-sided facial nerve palsy of six weeks duration. An ear swab culture was negative. He received ceftazidime and ciprofloxacin for 42 days. A complete resolution of the MOE was achieved after 29 sessions of HBOT. However, no improvement was noted with regard to the facial nerve palsy.
Case 17
A 60-year-old female with known uncontrolled diabetes and hypertension presented with a four-month history of persistent right ear pain, discharge, and right-sided facial nerve palsy. An ear swab culture showed beta-hemolytic group B Streptococcus. A CT scan confirmed temporal bone osteomyelitis. She received ceftazidime, piperacillin/tazobactam, and meropenem for 125 days. The patient underwent a total of 40 sessions of HBOT, which resulted in the complete resolution of the MOE and partial improvement of the facial nerve palsy.
Case 18
A 55-year-old female with uncontrolled diabetes and hypertension presented with persistent left ear pain and discharge of six weeks duration. P. aeruginosa was isolated from an ear swab culture. A CT scan revealed mastoiditis and the formation of cholesteatoma. The patient received ceftazidime and ciprofloxacin for 41 days. She underwent 27 sessions of HBOT and was cured of the disease.
Case 19
A 64-year-old male with controlled diabetes and hypertension presented with a four-week history of persistent right ear pain and discharge. An ear swab culture showed growth of P. aeruginosa and P. mirabilis. Mastoiditis was observed on CT imaging. The patient received ceftazidime and ciprofloxacin for 27 days and underwent 13 sessions of HBOT, with full recovery confirmed upon follow-up.
Case 20
A 62-year-old female with uncontrolled diabetes and hypertension presented with a two-month history of persistent right ear pain and discharge. An ear swab culture showed P. aeruginosa isolates. A CT scan confirmed temporal bone osteomyelitis. She received ceftazidime for 61 days and had 33 sessions of HBOT, resulting in a full recovery.
Discussion
According to a recent systematic review, the most common symptom of MOE is otorrhea (84.1%) followed by otalgia (77.3%), while common signs of disease in the EAC include granulation tissue (59.1%) and edema (27.3%).10 Similarly, all the patients in our case series had chronic otorrhea, with severe otalgia reported in 95.0% of patients, granulation tissue in 75.0%, and edema of the EAC in 35.0%. Usually, the disease originates in the EAC and spreads through the osseocartilaginous junction to the soft tissues beneath the temporal bone.9,11 Spreading infection can lead to various complications, such as skull base osteomyelitis. Furthermore, progressive osteomyelitis can result in cranial polyneuropathy.1,5 In our series, 70.0%, 25.0%, and 5.0% of patients had stage 2, stage 3a, and stage 4 disease, respectively [Table 1].3
Table 1: Disease staging of malignant otitis
externa (MOE).3
|
1
|
Clinical evidence of MOE with infection of the soft tissues beyond the EAC, but negative Tc-99 bone scan findings
|
|
2
|
Soft tissue infection beyond the EAC with positive Tc-99 bone scan findings
|
|
3a
|
As above, but with paralysis of a single cranial nerve
|
|
3b
|
As above, but with paralysis of multiple cranial nerves
|
EAC: external auditory canal; Tc-99: technetium-99.
Previous research indicates that facial nerve palsy in MOE is associated with a poorer prognosis.3 However, other researchers have failed to show an association between the involvement of the cranial nerves and survival.6,12 Similarly, there was no significant difference found in the survival of MOE patients with and without facial paralysis.13 In the present case series, four patients (20.0%) had palsy of the VII cranial nerve. By the end of the follow-up period, two of the patients showed no improvement, one had experienced partial recovery, and one patient had recovered completely. Another case series reported facial nerve palsy in four MOE patients (60%); of these, three underwent HBOT after conventional medical treatment failed, with improvement noted in two patients.5 These findings support the theory that not all facial nerve function recovers in MOE patients even when there is no evidence of continuing infection.3
One of the patients in our series had palsy of multiple cranial nerves, including the glossopharyngeal (IX), vagus (X), spinal accessory (XI), and hypoglossal (XII) nerves. Together, palsy of the former three cranial nerves is known collectively as jugular foramen syndrome. Nevertheless, despite the involvement of multiple cranial nerves, the patient improved and was disease-free after receiving adjunctive HBOT. This supports previous findings indicating that multiple cranial nerve involvement is not linked to survival.14
A marked elevation in erythrocyte sedimentation rate (ESR) is a useful marker to support a diagnosis of MOE and monitor therapeutic response.2,5 In our series, 95.0% of patients had a high ESR upon presentation; moreover, while one patient had no documented ESR, his C-reactive protient levels were high. Subsequently, among the 15 patients whom follow-up ESR measurements were collected, 14 returned to normal levels following HBOT. The remaining patient remained symptomatic for MOE with a persistently high ESR during the six months of follow-up until they had DOC. Another case series investigating patients with MOE reported that serial ESR measurements correlated well with the patients’ condition. Moreover, following HBOT, all patients improved clinically with a reduction in ESR.8
Although P. aeruginosa is the most isolated organism in MOE, other researchers have reported an increased frequency of other causative organisms.3 One study identified P. aeruginosa in 11 patients (55%) and S. aureus in three patients (12%).2 Our analysis showed P. aeruginosa isolates in 11 (44.0%) swab cultures, with P. mirabilis, K. pneumoniae, S. aureus, and group B Streptococcus isolated in several other cases. A. flavus was identified in a single patient in our series; this is a pathogen rarely isolated in MOE.2 In addition, we identified one patient with a mixed bacterial and fungal infection and seven patients with negative ear swab cultures. However, most patients in the current case series received oral empiric antibiotics before being seen at referring hospitals. Harris et al,15 found that bacteria were less often detected in culture-based tests collected after antibiotic use. In addition, some cultures may have been reported as negative due to the absence of data prior to admission.
When MOE was first described, it had a poor prognosis with > 50% mortality rate.9 Since then, the mortality rate has improved considerably, an improvement largely attributable to advances in treatment.12 A number of antimicrobial regimes for MOE have been documented in the literature, including prolonged courses of ciprofloxacin, meropenem, piperacillin/tazobactam, ceftazidime, and gentamicin.1,3 The recommended duration of antibiotic use in MOE treatment is six weeks and sometimes it is continued for several months depending on the improvement in imaging and physical examination due to poor perfusion of diseased areas.1,9 The specific choice of antibiotic depends upon hospital policy and microbiological cultures. Most of the patients in our case series received ciprofloxacin followed by ceftazidime as strong anti-pseudomonal agents. Oral ciprofloxacin is the preferred first-line treatment for MOE in an outpatient setting; however, due to the increased use of this drug, there is concern regarding the possibility of antibiotic resistance in Pseudomonas-associated infections. Nevertheless, such patients do not have an increased risk of disease-specific mortality.1,12
In our case series, one patient was found to have a Pseudomonas-associated infection that was resistant to multiple drugs, including ciprofloxacin. Although a swab culture sensitivity assessment informed the use of colistin, the patient did not show any improvement and had DOC within six months as a result of a myocardial infarction. Colistin has been reported to be an effective treatment for various infections caused by MDR P. aeruginosa, including in one case of bilateral MOE.12 Unfortunately, the patient in this case subsequently had DOC one year after diagnosis with no change in his chronic infection despite a prolonged antibiotic regimen.12
To the best of our knowledge, no previous research has yet investigated the therapeutic value of HBOT in MOE caused by MDR P. aeruginosa. The underlying theoretical principle of HBOT relies on the increase in oxygen supply and arterial oxygen tension.8 Inside the hyperbaric chamber, under typical therapeutic conditions, levels of partial pressure of oxygen dissolved in plasma can become > 20 times that of those observed when breathing room air at normal atmospheric pressure. The theory of action is that HBOT causes a decrease in the production of local nitric oxide by the endothelial cells, thereby leading to vasoconstriction and a decrease in edema of the damaged tissues. Moreover, increased arterial oxygen tension promotes modulation of several growth factors, proliferation of fibroblasts, activation of neoangiogenesis, and improved osteoblastic activity leading to enhanced healing.3,9
Oxygen also has antibacterial effects at the infection site. As neutrophils and macrophages enter these environments to kill bacteria, they consume large amounts of oxygen, which is then utilized by these cells to produce reactive oxygen species (ROSs). These ROSs can kill bacteria through membrane disruption and protein denaturation. Therefore, HBOT can enhance the response of the immune system to the infection. Interestingly, HBOT has even been found to increase the antibacterial effectiveness of certain antibiotics, such as aminoglycosides.4,6 Thus, employing HBOT for conditions that share a similar pathophysiology appears logical.8 Indeed, 95.0% of the patients in our case series were treated successfully with HBOT, with no died of disease cases and only one patient who had DOC.
Nevertheless, MOE is an uncommon disease that is difficult to diagnose; moreover, HBOT can be extremely slow and accessibility to hyperbaric chambers is often restricted. These limitations hinder the ability of researchers to conduct prospective, randomized, double-blind clinical trials to confirm the safety and efficacy of HBOT as a treatment modality for MOE.4 This may explain why most related publications in this area are based on the authors’ own practice and experiences. A retrospective study demonstrated disease resolution in 16 patients with MOE who received HBOT in conjunction with antibiotics after all other treatments had failed.3 The researchers reported that the duration of hospitalization prior to HBOT was significantly longer than the time between initiation of HBOT and cure (p = 0.028).3 In our study, the length of receiving treatment in the referring hospital prior to receiving HBOT was significantly associated with the number of HBOT sessions required for full recovery (p = 0.029). These findings point toward the need for the early initiation of HBOT.
Side effects of HBOT include ear barotrauma, sinus squeeze, dental problems, seizures, claustrophobia, and pulmonary oxygen toxicity; however, such effects are generally rare or transient in nature.16 In our case series, two patients reported middle ear barotrauma. Both were found to have had an upper respiratory tract infection prior to the therapeutic session, which made it difficult for them to equalize their ear pressure. They were treated with nasal decongestants and resumed HBOT treatment during their following scheduled sessions. Nasal decongestants have been shown to be effective in preventing middle ear barotrauma.17
Conclusion
The use of HBOT as adjuvant therapy in the management of MOE appears promising. Overall, 95% of the patients in this case series were considered cured by the end of the treatment. Early initiation of this treatment modality could prevent severe complications and reduce mortality associated with the disease. Moreover, early treatment may lessen the number of therapeutic sessions required for complete recovery, thereby reducing the need for prolonged hospitalization and related costs.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Illing E, Olaleye O. Malignant otitis externa: a review of aetiology, presentation, investigations and current management strategies. [cited 2021 May 4]. Available from: http://www.webmedcentral.com/article_view/1725.
- 2. Kaya İ, Sezgin B, Eraslan S, Öztürk K, Göde S, Bilgen C, et al. Malignant otitis externa: a retrospective analysis and treatment outcomes. Turk Arch Otorhinolaryngol 2018 Jun;56(2):106-110.
- 3. Amaro CE, Espiney R, Radu L, Guerreiro F. Malignant (necrotizing) externa otitis: the experience of a single hyperbaric centre. Eur Arch Otorhinolaryngol 2019 Jul;276(7):1881-1887.
- 4. Narozny W, Kuczkowski J, Stankiewicz C, Kot J, Mikaszewski B, Przewozny T. Value of hyperbaric oxygen in bacterial and fungal malignant external otitis treatment. Eur Arch Otorhinolaryngol 2006 Jul;263(7):680-684.
- 5. Karaman E, Yilmaz M, Ibrahimov M, Haciyev Y, Enver O. Malignant otitis externa. J Craniofac Surg 2012 Nov;23(6):1748-1751.
- 6. Davis JC, Gates GA, Lerner C, Davis MG Jr, Mader JT, Dinesman A. Adjuvant hyperbaric oxygen in malignant external otitis. Arch Otolaryngol Head Neck Surg 1992 Jan;118(1):89-93.
- 7. Singh A, Al Khabori M, Hyder MJ. Skull base osteomyelitis: diagnostic and therapeutic challenges in atypical presentation. Otolaryngol Head Neck Surg 2005 Jul;133(1):121-125.
- 8. Bath AP, Rowe JR, Innes AJ. Malignant otitis externa with optic neuritis. J Laryngol Otol 1998 Mar;112(3):274-277.
- 9. Shupak A, Greenberg E, Hardoff R, Gordon C, Melamed Y, Meyer WS. Hyperbaric oxygenation for necrotizing (malignant) otitis externa. Arch Otolaryngol Head Neck Surg 1989 Dec;115(12):1470-1475.
- 10. Byun YJ, Patel J, Nguyen SA, Lambert PR. Hyperbaric oxygen therapy in malignant otitis externa: a systematic review of the literature. World J Otorhinolaryngol Head Neck Surg 2020 May;7(4):296-302.
- 11. Bhargava D, Waite C, Hussein SS, Sankhla D, El Khatim HS, Woodhouse NJ. Malignant otitis externa and temporal bone osteomyelitis: complete recovery following the adjunctive use of hyperbaric oxygen and antibiotics. Sultan Qaboos Univ Med J 1999 Jan;1(1):47-50.
- Buelle H, Tucci VT, Greene JN, Vincent AL. Successful treatment of multi-drug resistant Pseudomonas aeruginosa, causing bilateral malignant otitis externa, media and mastoiditis. Asian Biomed 2009 Apr;3(2):159-164.
- 12. Simon Carney A. Malignant otitis externa. In: Gleeson M, editor. Scott–Brown’s otorhinolaryngology, head and neck surgery. 7th ed. Hodder Arnold, London; 2008. p. 3337-3341.
- 13. Mani N, Sudhoff H, Rajagopal S, Moffat D, Axon PR. Cranial nerve involvement in malignant external otitis: implications for clinical outcome. Laryngoscope 2007 May;117(5):907-910.
- 14. Harris AM, Bramley AM, Jain S, Arnold SR, Ampofo K, Self WH, et al. Influence of antibiotics on the detection of bacteria by culture-based and culture-independent diagnostic tests in patients hospitalized with community-acquired pneumonia. Open Forum Infect Dis 2017 Feb;4(1):ofx014.
- 15. Phillips JS, Jones SE. Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst Rev 2013 May;2013(5):CD004617.
- 16. Undersea and Hyperbaric Medical Society. Side effects. [cited 2021 May 4]. Available from: https://www.uhms.org/2-side-effects.html.