Since the onset of the COVID-19 pandemic in China in late 2019, millions of healthcare workers have died, and hundreds of millions of health personnel have been affected.1 SARS-CoV-2 mainly causes a severe respiratory disease;2 however, it has been reported to affect other systems such as the heart,3 kidneys,4 and gastrointestinal system.5 Vaccination is the best way to control and end this pandemic.6 Fortunately, several vaccines have been developed to prevent the further spread of this disease.7 The available vaccines are classified into four basic categories related to their production technologies: 1) inactivated virus, 2) viral vector, 3) protein-based, and 4) nucleic acid-based.8 The first dose of the Sputnik viral vaccine contains adenovirus 26. The second dose contains adenovirus 5 as a vector. These vectors carry the gene encoding spike protein, a coronavirus gene, to stimulate the host's immune system against the virus.9 All four vaccines are new. Despite their safety test results and government approval, people around the globe, especially healthcare workers are hesitant toward COVID-19 vaccination.10,11 Therefore, further assessment on greater scales of time and population is needed for public reassurance. Also, side effect monitoring helps the healthcare system and manufacturers ensure the quality of vaccines and follow subjects in the post-vaccination period. Scientists in different countries were sensitive and did their evaluations.12,13 Because the outbreak of vaccine side effects plays a critical role in public perception, especially the Iranian population, about vaccination programs in Iran, different studies have given different assessments on post-vaccination side effects.14,15 Covaxin and Sinopharm contain inactivated viruses to stimulate the immune system but do not cause disease.16 Studies have shown that vaccination effectively reduces the risk of severe disease, hospitalization, and mortality from COVID-19.9 However, post-vaccination side effects include fever, fatigue, muscle pain, skin rash and swelling,7 headache,17 restlessness, injection site pain and joint pain,18 purpura and erythematosus,19 and nausea and chills20 have been reported.
The objective of this study was to investigate the potential side effects of Sinopharm, AstraZeneca, Sputnik, and Covaxin vaccination and the relationship between the side effects and age, BMI, a previous history of COVID-19 infection, and allergies.
Methods
A descriptive-analytical cross-sectional study was conducted among 1474 healthcare workers working at seven selected hospitals in Tehran from June to August 2021. A healthcare worker was considered anyone who works in the studied hospitals, including healthcare students on clinical placement, and frontline healthcare workers (e.g., doctors, nurses, paramedics, medical technicians, laboratory technicians, etc.). Those employed as healthcare workers in one of the seven studied hospitals and had a history of at least one dose of the COVID-19 vaccine were included in the study. The exclusion criteria were no history of COVID-19 vaccination or employment in non-healthcare jobs. Data were collected by a researcher-made questionnaire using the complete enumeration method. The questionnaire consisted of 54 two-choice questions, five multiple-choice questions, and three open-ended questions. This study was approved by Aja university medical science (IR.AJAUMS.REC.1400.163). The study was performed by the principles of the Declaration of Helsinki.
Demographic data included gender, age, height, weight, marital status, underlying diseases such as hypertension, hyper- or hypothyroidism, kidney, heart, lung, and skin disease, diabetes, a history of allergic reaction to the influenza vaccine, a history of COVID-19 disease, the time from previous COVID-19 disease, the type of vaccine, and the number of doses received by the subjects. The side effects asked in the questionnaire included topical reactions (pain, swelling, and itching at the site of injection), fever, itchy skin, shortness of breath, numbness in the face, body, arms, and legs, swelling in the face, mouth or whole body, anaphylactic shock, fatigue, cold-like symptoms, muscle pain, chills, swelling in legs, nausea, vomiting, abdominal pain, diarrhea, sore throat, severe decrease in platelet count and coagulation problems, joint pain, excessive sweating, dizziness, nasal congestion, pain when swallowing, poor sleep quality, purpura, palpitation, hot flashes, severe drowsiness, extreme hunger and thirst, feeling energized, restlessness (malaise), hives, hair loss, eye soreness, runny nose, chest tightness, lip swelling, cough, balance problems, hematuria (blood in the urine), anorexia, delirium, olfactory, visual, hearing, and sexual dysfunctions. Also, the average time of the incidence of side effects, the need to go to an emergency department or medical center due to the vaccine’s side effects, as well as the duration of maximum inefficiency or difficulty in performing daily tasks after vaccination, and getting back to feel healthy enough after vaccination to be able to perform his/her healthcare activities in the hospital properly without any problems were assessed.
The questionnaire questions were prepared based on valid documents from the World Health Organization and Centers for Disease Control and Prevention.21,22 Then, the instrument was carefully examined by 10 faculty members, clinical infections, and allergy immunity specialists, and the necessary changes were made. For the validity of the questionnaire, the questions were evaluated using the content validity index (CVI), the questions with CVI < 0.7 were omitted, and with CVI between 0.7–0.79 were reviewed. To confirm the reliability, the internal consistency was examined using Cronbach’s alpha (α) coefficient, and good reliability (α = 0.89) was found.
The researcher obtained the ethical approval (approval ID: IR.AJAUMS.REC.1400.163), entered the medical centers, and distributed the questionnaire among healthcare workers who met the inclusion criteria. Before distributing the questionnaires, the study’s objectives were explained, the confidentiality of the subjects was ensured, and the issues were signed on a consent form. The collected data were analyzed using SPSS (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.). To analyze the data, central tendency and dispersion indicators including mean, SD, tables and diagrams, and the chi-squared, Fisher’s exact, one-sample Kolmogorov-Smirnov, Mann-Whitney U, and t-tests were used. The level of significance was considered < 0.05.
Results
The mean age of the participants was 26.2±9.0 years. There were 1039 men (70.5%) and 435 women (29.5%), of whom 123 received one dose, and 1351 (91.7%) received two doses of the COVID-19 vaccine. The majority (91.7%) had no underlying disease; 36.0% (n = 530) had COVID-19 infection. Eight hundred seventy-six healthcare workers (59.4%) were aged 20–29 years old. The mean BMI of the subjects was 33.6±3.4, with 983 (66.7%) health personnel showing normal BMI and 86 (5.8%) were obese, 350 (23.7%) participants were overweight, and 45 (3.1%) participants were under weight. Of 1474 health personnel, 890 (60.4%) received the Sinopharm, 309 (21.0%) received the AstraZeneca, 214 (14.5%) received the Sputnik, and 61 (4.1%) received Covaxin vaccines [Table 1].
Table 1: Demographic characteristics of participants (N = 1474).
|
Sex
|
|
Male
|
132(61.7)
|
27 (44.3)
|
192 (62.1)
|
688 (77.3)
|
|
Female
|
82 (38.3)
|
34 (55.7)
|
117 (37.9)
|
202 (22.7)
|
|
Marital status
|
|
Single
|
80 (37.4)
|
20 (32.8)
|
128 (41.4)
|
802 (90.1)
|
|
Married
|
134 (62.6)
|
41 (67.2)
|
181 (58.6)
|
88 (9.9)
|
|
Position
|
|
|
|
|
|
|
|
|
|
Doctor
|
34 (159)
|
11 (18.0)
|
53 (17.2)
|
102 (11.5)
|
|
Nurse
|
71 (33.2)
|
22 (36.1)
|
118 (38.2)
|
305 (34.3)
|
|
Technician
|
109 (51.0)
|
28 (45.9)
|
138 (44.7)
|
483 (54.3)
|
|
Number of received dose
|
|
One
|
22 (10.3)
|
1 (1.6)
|
85 (27.5)
|
15 (1.7)
|
|
Two
|
192 (89.7)
|
60 (98.4)
|
224 (72.5)
|
875 (98.3)
|
|
History of underlying disease
|
|
No disease
|
191 (89.3)
|
53 (86.9)
|
271 (87.8)
|
837 (94.0)
|
|
Hypertension
|
1 (0.5)
|
3 (4.9)
|
6(1.9)
|
11 (1.2)
|
|
Hypo- or hyperthyroidism
|
3 (1.4)
|
1 (1.6)
|
9 (2.9)
|
14 (1.6)
|
|
Allergy
|
4 (1.9)
|
1 (1.6)
|
6 (1.9)
|
5 (0.6)
|
|
Neurological disease
|
2 (0.9)
|
0 (0.0)
|
0 (0.0)
|
1 (0.1)
|
|
Kidney disease
|
2 (0.9)
|
0 (0.0)
|
0 (0.0)
|
2 (0.2)
|
|
Lung disease
|
0 (0.0)
|
0 (0.0)
|
3 (1.0)
|
0 (0.0)
|
|
Diabetes
|
4 (1.9)
|
0 (0.0)
|
6 (1.9)
|
3 (0.3)
|
|
Skin disease
|
1 (0.5)
|
1 (1.6)
|
1 (0.3)
|
5 (0.6)
|
|
Heart disease
|
1 (0.5)
|
0 (0.0)
|
2 (0.6)
|
1 (0.1)
|
|
Liver disease
|
0 (0.0)
|
0 (0.0)
|
1 (0.3)
|
2 (0.2)
|
|
Addiction
|
2 (0.9)
|
1 (1.6)
|
1 (0.3)
|
3 (0.3)
|
|
Others
|
3 (1.4)
|
1 (1.6)
|
3 (1.0)
|
6 (0.7)
|
|
History of allergy to influenza vaccine
|
12 (5.6)
|
3 (9.4)
|
6 (1.9)
|
7 (0.8)
|
|
Previous COVID-19 Infection
|
95 (44.4)
|
18 (29.5)
|
106 (34.3)
|
311 (34.9)
|
|
Date of previous COVID-19 infection
|
|
|
|
|
|
|
|
|
|
No previous COVID-19 infection
|
119 (55.6)
|
43 (70.5)
|
204 (66.0)
|
561 (63.0)
|
|
1 month before vaccination
|
9 (4.2)
|
0 (0.0)
|
5 (1.6)
|
12 (1.3)
|
|
2–3 months before vaccination
|
14 (6.5)
|
1 (1.6)
|
13 (4.2)
|
71 (8.0)
|
|
4–6 months before vaccination
|
29 (13.6)
|
7 (11.5)
|
33 (10.7)
|
90 (10.1)
|
|
> 6 months before vaccination
|
43 (20.1)
|
10 (16.4)
|
54 (17.5)
|
156 (17.5)
|
|
Age, years
|
|
|
|
|
|
|
|
|
|
< 20
|
16 (7.5)
|
0 (0.0)
|
2 (0.6)
|
137 (15.4)
|
|
20–29
|
64 (29.9)
|
23 (37.7)
|
121 (39.2)
|
668 (75.1)
|
|
30–39
|
76 (35.5)
|
19 (31.1)
|
80 (25.9)
|
39 (4.4)
|
|
40–49
|
44 (20.6)
|
6 (9.8)
|
85 (27.5)
|
28 (3.1)
|
|
50–59
|
12 (5.6)
|
13 (21.3)
|
13 (4.2)
|
10 (1.1)
|
|
> 50
|
2 (0.9)
|
0 (0.0)
|
8 (2.6)
|
8 (0.9)
|
|
Body Mass Index
|
|
Underweight (< 18.5)
|
8 (3.7)
|
2 (3.3)
|
8 (2.6)
|
27 (3.0)
|
|
Normal (18.5–25)
|
110 (51.4)
|
41 (67.2)
|
169 (54.7)
|
663 (74.5)
|
|
Overweight (25–29.9)
|
79 (36.9)
|
16 (25.8)
|
92 (29.8)
|
163 (18.3)
|
The results showed no side effects in 622 subjects (42.2%) after vaccination for all four vaccines [Table 2]. It was found that 93.9% of the subjects received AstraZeneca, 86.0% received Sputnik, 77.0% received Covaxin, and 37.2% received Sinopharm showed at least one complication after vaccination. The lowest and highest occurrence rates of side effects were observed for Sinopharm and AstraZeneca vaccines, respectively. There was a significant (p < 0.001) difference in the occurrence of at least one side effect among the vaccines [Figure 1].
Table 2: Frequency of at least one side effect and referral to medical centers among subjects receiving AstraZeneca, Sputnik, Covaxin, and Sinopharm vaccines.
|
Occurrence of at least one side effect
|
|
Yes
|
290 (93.9)
|
184 (86.0)
|
47 (77.0)
|
331 (37.2)
|
852 (57.8)
|
< 0.001
|
|
No
|
19 (6.1)
|
30 (14.0)
|
14 (23.0)
|
559 (62.8)
|
622 (42.2)
|
|
|
Referral to the medical center
|
|
Yes
|
77 (24.9)
|
39 (18.2)
|
9 (14.8)
|
31 (3.5)
|
156 (10.6)
|
< 0.001
|
|
no
|
232 75.1)
|
175 (81.8)
|
52 (85.2)
|
859 (96.5)
|
1318 (89.4)
|
|
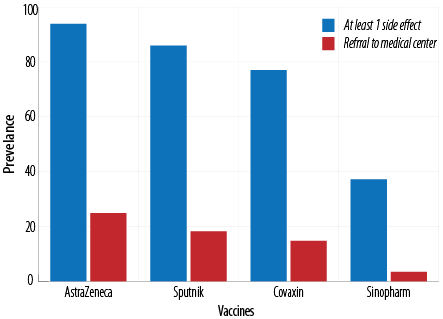 Figure 1: Frequency of at least one side effect and referral to medical centers among subjects receiving AstraZeneca, Sputnik, Covaxin, and Sinopharm.
Figure 1: Frequency of at least one side effect and referral to medical centers among subjects receiving AstraZeneca, Sputnik, Covaxin, and Sinopharm.
The need to refer to medical centers was also investigated. It was shown that 24.9% of healthcare workers receiving the AstraZeneca vaccine had a complication that required referral to a medical center or a hospital for services such as intravenous therapy. The rates of referral to a medical center for Sputnik, Covaxin, and Sinopharm were 18.2%, 14.8%, and 3.5%, respectively, showing a significant (p < 0.001) difference [Table 2].
Fever was the most common complication after vaccination, with the complication rates for AstraZeneca, Sputnik, Covaxin, and Sinopharm being 64.4%, 49.5%, 49.2%, and 18.7%, respectively. The second most common complication was fatigue, with the complication rates for AstraZeneca, Sputnik, Covaxin, and Sinopharm being 62.5%, 46.7%, 34.4%, and 16.6%, respectively. The most common post-vaccination side effect was muscle pain followed by topical reaction, chills, dizziness, headache, and cold-like symptoms. The rate of other complications, including poor sleep quality, shortness of breath, palpitation, runny nose, etc., was less than 1.5% for all four vaccines. Sinopharm and AstraZeneca had the least and the most side effects among the four studied vaccines [Table 3 and Figure 2].
Table 3: Frequency of side effects of four studied vaccines. Data are given as n (%).
|
Fever
|
199 (64.4)
|
106 (49.5)
|
30 (49.2)
|
166 (18.7)
|
|
Fatigue
|
193 (62.5)
|
100 (46.7)
|
21 (34.4)
|
148 (16.6)
|
|
Muscle pain
|
185 (59.9)
|
128 (59.8)
|
19 (31.1)
|
91 (10.2)
|
|
Topical reaction
|
113 (36.6)
|
57 (26.6)
|
25 (41.0)
|
159 (17.9)
|
|
Chills
|
161 (52.1)
|
89 (41.6)
|
14 (23.0)
|
37 (4.2)
|
|
Dizziness or headache
|
80 (25.9)
|
47 (22.0)
|
11 (18.0)
|
85 (9.6)
|
|
Cold-like symptoms
|
113 (36.6)
|
49 (22.9)
|
10 (16.4)
|
39 (4.4)
|
|
Poor sleep quality
|
39 (12.6)
|
22 (10.3)
|
3 (4.9)
|
24 (2.7)
|
|
Excessive sweating
|
42 (13.6)
|
22 (10.3)
|
5 (8.2)
|
12 (1.3)
|
|
Joint pain
|
38 (12.3)
|
24 (11.2)
|
3 (4.9)
|
10 (1.1)
|
|
Shortness of breath
|
25 (8.1)
|
7 (3.3)
|
2 (3.3)
|
14 (1.6)
|
|
Nasal congestion
|
20 (6.5)
|
10 (4.7)
|
0 (0.0)
|
18 (2.0)
|
|
Palpitation
|
15 (4.9)
|
13 (6.1)
|
2 (3.3)
|
10 (1.3)
|
|
Itchy skin
|
14 (4.5)
|
6 (2.8)
|
3 (4.9)
|
19 (2.1)
|
|
Nausea
|
19 (6.1)
|
4 (1.9)
|
6 (9.8)
|
8 (0.9)
|
|
Numbness (face and extremities)
|
9 (2.9)
|
6 (2.8)
|
3 (4.9)
|
15 (1.7)
|
|
Sore throat
|
12 (3.9)
|
12 (5.6)
|
1 (1.6)
|
8 (0.9)
|
|
Diarrhea
|
14 (4.5)
|
4 (1.9)
|
2 (3.3)
|
9 (1.0)
|
|
Abdominal pain
|
11 (3.6)
|
4 (1.9)
|
1 (1.6)
|
7 (0.8)
|
|
Olfactory and taste dysfunction
|
6 (1.9)
|
4 (1.9)
|
2 (3.3)
|
8 (0.9)
|
|
Pain when swallowing
|
9 (2.9)
|
8 (3.7)
|
0 (0.0)
|
2 (0.2)
|
|
Purpura
|
10 (3.2)
|
0 (0.0)
|
1 (1.6)
|
3 (0.3)
|
|
Anaphylactic shock
|
6 (1.9)
|
1 (0.5)
|
0 (0.0)
|
6 (0.7)
|
|
Vomiting
|
6 (1.9)
|
1 (0.5)
|
3 (4.9)
|
2 (0.2)
|
|
Coagulation problems and a decrease in platelet count
|
7 (2.3)
|
4 (1.9)
|
0 (0.0)
|
0 (0.0)
|
|
Hot flashes
|
2 (0.6)
|
4 (1.9)
|
0 (0.0)
|
5 (0.6)
|
|
Visual dysfunction
|
2 (0.6)
|
5 (2.3)
|
0 (0.0)
|
3 (0.3)
|
|
Severe drowsiness
|
1 (0.3)
|
1 (0.5)
|
0 (0.0)
|
7 (0.8)
|
|
Feeling energized
|
6 (1.9)
|
1 (0.5)
|
0 (0.0)
|
0 (0.0)
|
|
Restlessness (malaise)
|
1 (0.3)
|
0 (0.0)
|
1 (1.6)
|
4 (0.4)
|
|
Swelling (face)
|
2 (0.6)
|
2 (0.9)
|
0 (0.0)
|
1 (0.1)
|
|
Constant hunger and thirst
|
1 (0.3)
|
0 (0.0)
|
0 (0.0)
|
4 (0.4)
|
|
Hives
|
1 (0.3)
|
0 (0.0)
|
0 (0.0)
|
3 (0.3)
|
|
Hearing dysfunction
|
2 (0.6)
|
2 (0.9)
|
0 (0.0)
|
0 (0.0)
|
|
Hair loss
|
0 (0.0)
|
1 (0.5)
|
0 (0.0)
|
2 (0.2)
|
|
Eye soreness
|
1 (0.3)
|
1 (0.5)
|
0 (0.0)
|
1 (0.1)
|
|
Runny nose
|
1 (0.3)
|
0 (0.0)
|
2 (3.3)
|
0 (0.0)
|
|
Chest tightness
|
0 (0.0)
|
1 (0.5)
|
1 (1.6)
|
1 (0.1)
|
|
Lip swelling
|
1 (0.3)
|
0 (0.0)
|
2 (3.3)
|
0 (0.0)
|
|
Cough
|
0 (0.0)
|
1 (0.5)
|
0 (0.0)
|
2 (0.2)
|
|
Swelling (legs)
|
1 (0.3)
|
0 (0.0)
|
0 (0.0)
|
1 (0.1)
|
|
Balance problems
|
2 (0.6)
|
0 (0.0)
|
0 (0.0)
|
0 (0.0)
|
|
Hematuria
|
1 (0.3)
|
0 (0.0)
|
0 (0.0)
|
1 (0.1)
|
|
Anorexia
|
1 (0.3)
|
1 (0.5)
|
0 (0.0)
|
0 (0.0)
|
|
Sexual dysfunction
|
0 (0.0)
|
1 (0.5)
|
0 (0.0)
|
1 (0.1)
|
|
Hallucination
|
0 (0.0)
|
1 (0.5)
|
0 (0.0)
|
0 (0.0)
|
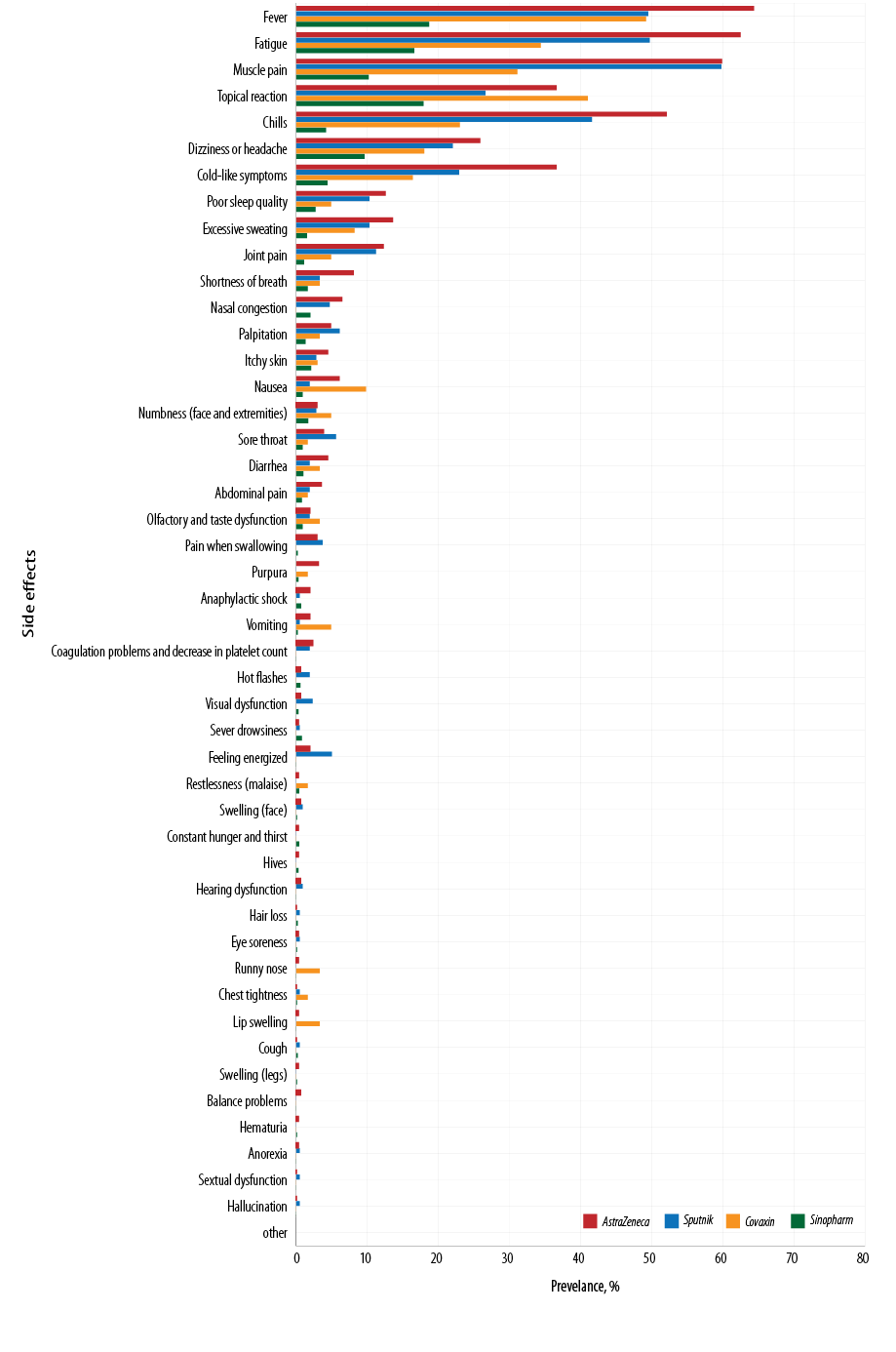 Figure 2: Frequency of 47 adverse effects following Sinopharm, Covaxin, Sputnik, and AstraZeneca vaccination.
Figure 2: Frequency of 47 adverse effects following Sinopharm, Covaxin, Sputnik, and AstraZeneca vaccination.
The seven most common post-vaccination side effects were reported in the following order:
- AstraZeneca: fever, fatigue, muscle pain, chills, topical reaction, cold-like symptoms, and dizziness or headache.
- Sputnik: muscle pain, fever, fatigue, chills, topical reaction, cold-like symptoms, and dizziness or headache.
- Covaxin: fever, topical reaction, fatigue, muscle pain, chills, cold-like symptoms, and dizziness or headache.
- Sinopharm: fever, topical reaction, fatigue, muscle pain, dizziness or headache, cold-like symptoms, and chills.
Side effects happened within the first 24 hours after vaccination among 72.5% receiving AstraZeneca, 72.9% of those receiving Sputnik, 57.4% receiving Covaxin, and 26.6% receiving Sinopharm [Table 4 and Figure 3].
Table 4: Time of occurrence of side effects.
|
No side effects
|
19 (6.1)
|
30 (14.0)
|
14 (23.0)
|
559 (62.8)
|
622 (42.2)
|
|
< 24
|
223 (72.5)
|
156 (72.9)
|
35 (57.4)
|
237 (26.6)
|
651 (44.2)
|
|
24–47
|
54 1(7.5)
|
22 (10.3)
|
8 (13.1)
|
56 (6.3)
|
140 (9.5)
|
|
48–71
|
6 (1.9)
|
2 (0.9)
|
1 (1.6)
|
18 (2.0)
|
27 (1.8)
|
|
> 72
|
7 (2.3)
|
4 (1.9)
|
3 (4.9)
|
20 (2.2)
|
34 (2.3)
|
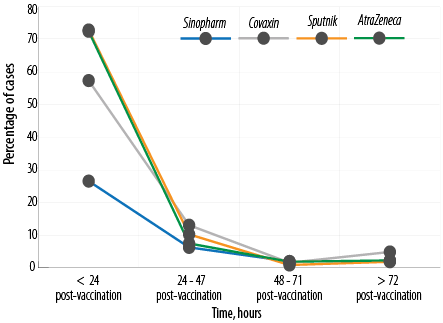 Figure 3: Time of occurrence of side effects.
Figure 3: Time of occurrence of side effects.
The ability of the subjects to perform daily tasks after vaccination with the four studied vaccines was investigated: 58.3% of those receiving AstraZeneca, 40.7% receiving Sputnik, 29.5% receiving Covaxin, and 13.5% receiving Sinopharm reported that they could not perform their daily tasks within 24 hours after vaccination and needed to rest [Figure 4 and Table 5].
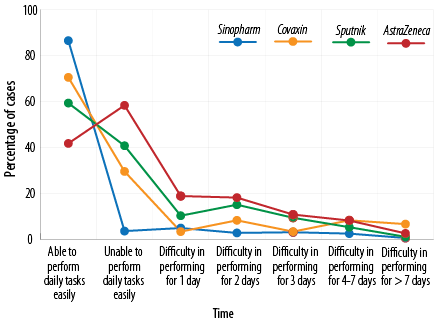 Figure 4: The rate of inefficiency after vaccination.
Figure 4: The rate of inefficiency after vaccination.
Table 5: The rate of inefficiency after vaccination.
|
Able to perform daily tasks easily
|
129 (41.7)
|
127 (59.3)
|
43 (70.5)
|
720 (80.9)
|
|
Unable to perform daily tasks easily
|
180 (58.3)
|
87 (40.7)
|
18 (29.5)
|
120 (13.5)
|
|
Difficulty in performing for one day
|
8 (2.6)
|
23 (10.7)
|
2 (3.3)
|
43 (4.8)
|
|
Difficulty in performing for two days
|
56 (18.1)
|
32 (15.0)
|
5 (8.2)
|
24 (2.7)
|
|
Difficulty in performing for three days
|
33 (10.7)
|
20 (9.3)
|
2 (3.27)
|
27 (3.0)
|
|
Difficulty in performing for 4–7 days
|
25 (8.1)
|
11 (5.1)
|
5 (8.2)
|
21 (2.4)
|
The relationship between the subjects’ age and BMI and post-vaccination side effects was studied, and no significant relationship was observed for Sputnik, Covaxin, and AstraZeneca. The side effects of the Sinopharm vaccine were significantly higher among younger healthcare workers [Table 6]. There was no significant relationship between BMI and the occurrence of side effects for the four studied vaccines.
Table 6: Age of subjects with or without post-vaccination side effects.
|
AstraZeneca
|
Positive
|
36.5 ± 5.9
|
0.474
|
Positive
|
25.1 ± 4.4
|
0.955
|
|
Negative
|
34.4 ± 9.9
|
|
Negative
|
25.0 ± 3.7
|
|
|
Sputnik
|
Positive
|
34.7 ± 5.5
|
0.474
|
Positive
|
24.4 ± 2.5
|
0.629
|
|
Negative
|
34.1 ±9.6
|
|
Negative
|
24.9 ± 3.7
|
|
|
Covaxin
|
Positive
|
29.7 ± 6.1
|
0.188
|
Positive
|
21.3 ± 2.2
|
0.166
|
|
Negative
|
35.7 ± 9.8
|
|
Negative
|
24.3 ± 3.2
|
|
|
Sinopharm
|
Positive
|
21.7 ± 4.4
|
< 0.001
|
Positive
|
22.9± 2.8
|
0.388
|
The frequency of side effects was higher among subjects with a previous history of COVID-19 disease for all the four studied vaccines; however, there was no significant relationship between the prior history of COVID-19 and the frequency of side effects for Sputnik, Covaxin, and AstraZeneca vaccines [Table 7]. However, side effects were significantly higher among those receiving Sinopharm with a history of COVID-19.
Table 7: Frequency of side effects among subjects with or without a history of COVID-19 and allergy to influenza vaccine. Data are given as n (%).
|
Previous COVID-19 disease
|
|
Yes
|
102 (96.2)
|
4 (3.8)
|
106 (100)
|
85 (89.5)
|
10 (10.5)
|
95 (100)
|
16 (88.9)
|
2 (11.1)
|
18 (100)
|
142 (45.7)
|
169 (54.3)
|
311 (100)
|
|
No
|
188 (92.6)
|
15 (7.4)
|
203 (100)
|
99 (83.2)
|
20 (16.8)
|
119 (100)
|
31 (72.1)
|
12 (27.9)
|
43 (100)
|
189 (32.6)
|
390 (67.4)
|
579 (100)
|
|
p-value
|
0.209
|
0.189
|
0.197
|
< 0.001
|
|
Allergy to influenza vaccine
|
|
Yes
|
6 (100)
|
0 (0.0)
|
6 (100)
|
9 (75.0)
|
3 (25.0)
|
12 (100)
|
3 (100)
|
0 (0.0)
|
3 (100)
|
6 (85.7)
|
1 (14.3)
|
7 (100)
|
|
No
|
283 (93.7)
|
19 (6.3)
|
302 (100)
|
175 (86.6)
|
27 (13.4)
|
202 (100)
|
44 (77.2)
|
13 (22.8)
|
57 (100)
|
325 (36.8)
|
558 (63.2)
|
883 (100)
|
We found no significant relationship between a history of COVID-19 and the need to refer to medical centers. Also, the results showed no significant relationship between a previous history of allergy to the influenza vaccine and the occurrence of side effects among those receiving Sputnik, Covaxin, and AstraZeneca vaccines. Only among the subjects receiving Sinopharm had a significant relationship between a history of allergy and the occurrence of side effects (p = 0.012) [Table 7].
The studied vaccines were divided into viral vector vaccines (Sputnikand AstraZeneca) and inactivated virus vaccines (Covaxin and Sinopharm); 90.6% of those receiving viral vector vaccines and 39.7% of those receiving inactivated virus vaccines reported the occurrence of side effects [Table 8].
Table 8: Comparison of occurrence of side effects and referral to medical centers for inactivated virus vaccines (Sinopharm and Covaxin) and viral vector vaccines (Sputnik and AstraZeneca).
|
Occurrence of at least one side effect
|
|
Yes
|
474 (90.6)
|
523 (100)
|
378 (39.7)
|
951(100)
|
852 (57.8)
|
1474 (100)
|
< 0.001
|
|
No
|
49 (9.4)
|
|
573 (60.3)
|
|
622 (42.4)
|
|
|
|
Referral to medical centers
|
|
Yes
|
116 (22.2)
|
523 (100)
|
40 (4.2)
|
951 (100)
|
156 (10.6)
|
1474 (100)
|
< 0.001
|
In addition, 22.2% of subjects vaccinated with viral vector vaccines and 4.2% of those immunized with inactivated virus vaccines needed a referral to medical centers. The occurrence of at least one side effect and referral to medical centers for the viral vector group was significantly (p < 0.001) higher than those for inactivated virus vaccines [Figure 5].
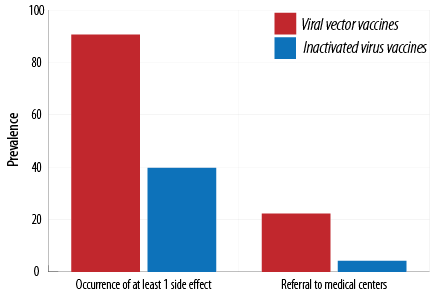 Figure 5: Comparison of occurrence of side effects and referral to medical centers for inactivated virus vaccines (Sinopharm and Covaxin) and viral vector vaccines (Sputnik and AstraZeneca).
Figure 5: Comparison of occurrence of side effects and referral to medical centers for inactivated virus vaccines (Sinopharm and Covaxin) and viral vector vaccines (Sputnik and AstraZeneca).
Discussion
We investigated the post-vaccination side effects of four vaccines among the medical care team members of seven selected hospitals in Tehran, Iran.
The results showed that 57.5% of the subjects experienced at least one side effect. The healthcare workers who received AstraZeneca and Sinopharm vaccines showed the most and the most minor frequency side effects, respectively. Our results are consistent with the results obtained in Iraq and Jordan.17,23
In a study on the side effects of the Sinopharm vaccine in the UAE, only 24.5%and 37.5% of Turkish healthcare staff did not report any side effects.15,16 However, our study showed that 62.8% of subjects reported no side effects. The need to refer to medical centers due to the side effects (generally temporary) they have faced after receiving the vaccine commonly fever and restlessness, or side effects that cause weakness and lethargy, and pressure reduction such as diarrhea, anorexia, and digestive side effects. We should also keep in mind that health personnel have better access to medical therapies (e.g., serious therapy) due to their profession. Frequent use of serum therapy might relate to the ease of access. This number for AstraZeneca, Sputnik, Covaxin, and Sinopharm vaccines was 24.9%, 18.2%, 14.8%, and 3.5%, respectively. However, these percentages were 3.2% and 0% for those who received the AstraZeneca vaccine in Germany and Ethiopia, respectively.24,25 Also, in a study conducted in the UAE, there was no post-vaccination complication among the subjects vaccinated with Sinopharm requiring hospitalization.26 Half of the Turkish healthcare staff receiving the Sinopharm vaccine needed to be referred to the hospital.27 The results showed that the most likely time for complications was the first 24 hours. On the second day for the AstraZeneca, Covaxin, and Sinopharm vaccines, this probability was about one-fourth of the chance on the first day and one-seventh for the Sinopharm vaccine, and then decreased significantly. A previous study showed that most complications such as fatigue, headache, and topical reaction occurred within the first 24 hours.27 About 60% of healthcare workers receiving the AstraZeneca vaccine in Ethiopia reported that topical reaction occurred 12 hours after vaccination and continued for another 24–72 hours.24
There was no significant relationship between age and occurrence of side effects for AstraZeneca, Sputnik, and Covaxin. However, the occurrence rate of side effects of Sinopharm was significantly higher among younger healthcare workers. Previous studies on the AstraZeneca vaccine in Germany25 and the Sinopharm vaccine in the UAE26 revealed that topical side effects were higher among younger healthcare workers. In contrast, systemic complications were higher among older healthcare workers. A study conducted in Iraq showed that the occurrence rate of side effects was higher among healthcare workers under 50 years old.23 A survey of the safety of the AstraZeneca vaccine in Brazil, Africa, and England showed a lower occurrence rate of side effects among older health personnel.28 Some studies on the AstraZeneca vaccine and the current study showed no significant relationship between the age and occurrence of post-vaccination side effects.7,29 There was no meaningful relationship between BMI and the occurrence of side effects in agreement with a study conducted in the UK.27 The occurrence rate of complications for Sputnik, Covaxin, and AstraZeneca was higher among those with a previous history of COVID-19 disease; however, they showed no significant relationship. Our result is consistent with the findings of a study conducted in Poland.30 However, the occurrence rate of side effects was significantly higher among those receiving the Sinopharm vaccine. A previous history of COVID-19 was consistent with a study conducted in Turkey.31 In a survey of the vaccines used in Iraq, the occurrence rate of complications was higher among healthcare workers with a history of COVID-19.23
The most common complication found among healthcare workers vaccinated with Sinopharm was fever, followed by fatigue, topical reaction, muscle pain, dizziness or headache, cold-like symptoms, and chills. In studies performed in Turkey and the UAE,26,31 injection site pain was the most common topical side effect. The most common systemic complications were fatigue, headache, and muscle and joint pain.
Previous studies showed that the most common side effects of the Sputnik vaccine were injection site pain followed by fever, fatigue, headache, lethargy, and muscle and joint pain.32,33 Our results revealed that the most common complication of Sputnik was muscle pain followed by fever, fatigue, chills, topical reaction, cold-like symptoms, and dizziness or headache. The most common side effects of the Covaxin vaccine were fever, topical reaction, fatigue, muscle pain, chills, cold-like symptoms, and dizziness or headache. Previous studies reported that the most common complications of Covaxin vaccines were tenderness, injection site pain, headache, muscle pain, nausea, and fatigue.29 The adverse effects of AstraZeneca were fever, fatigue, muscle pain, chills, topical reaction, cold-like symptoms, and dizziness or headache. Previous studies found that the most common complication of the AstraZeneca vaccine was fatigue, followed by headache, body aches, muscle pain, topical reaction, fever, and joint pain.34 In a study conducted in Iraq, the most common adverse effects of AstraZeneca were fever, fatigue, topical reaction, muscle pain, headache, and chills, and of Sinopharm were topical reaction, fatigue, fever, muscle pain, headache, and chills.23
Our results demonstrated that the occurrence rate of side effects of viral vector vaccines (AstraZeneca and Sputnik) was 90.6% and of inactivated virus vaccines (Sinopharm and Covaxin) was only 39.7%, which is in agreement with the results of studies conducted in Germany25 and England,7 which reported that the occurrence rate was higher for viral vector and messenger RNA vaccines. According to the European Medicines Agency report, the viral vector AstraZeneca vaccine leads to more side effects than inactivated virus Sinopharm vaccine.
The cause of such side effects trends in our study and the Iranian population is probably because the Iranian population is mainly vaccinated with four types of vaccines two of which are viral vectors. As previously mentioned, the most common side effects of viral-vector vaccines are inflammation-related and originate from factors such as tumor necrosis factor α, interleukin-1, and interleukin-6. Studies have shown that this kind of vaccine can bind with receptors like coxsackie and adenovirus receptor and toll-like receptor, leading to the secretion of inflammation factors. As a result, the vaccinated subject may present inflammation signs like fever, fatigue, muscle pain, and skin reaction, similar to the observed subjects in our study.35 The probable reason for fewer side effects of Sinopharm and Covaxin is because these vaccines are inactivated viruses, so they cause less inflammation and flu-like symptoms.8,16
Seven healthcare workers vaccinated with AstraZeneca (2.3%) and four vaccinated with Sputnik (1.9%) showed coagulation problems or decreased platelet count, previously found after viral vector vaccines.36,37 Out of 20 million health personnel who received the AstraZeneca vaccine in the UK, 79 cases of a decrease in platelet count were observed.38 However, a study conducted among 492 healthcare workers vaccinated with AstraZeneca observed no thrombocytopenia (low platelet count).39
Kadali et al,40 performed a study among health personnel vaccinated with the Moderna vaccine. They found that 79.7% could do their daily activities on the first day after vaccination.The percentage found in our study was 41.7% for AstraZeneca, 59.3% for Sputnik, 70.5% for Covaxin, and 80.9% for Sinopharm vaccines.
The number of health personnel who received Covaxin and Sputnik vaccines was lower than those receiving other vaccines. The number of women was lower than that of men. It should be noted that since all of the subjects were healthcare workers, with the majority being hospital staff, they received different therapies, (e.g., intravenous drip therapy) simply because of the ease of access while not an urgent need.
As we know for the first time, a wide range of post-vaccination side effects (n = 47) of Sinopharm, AstraZeneca, Sputnik, and Covaxin and the impact of various factors such as age, BMI, a previous history of COVID-19, and a previous history of allergy to influenza vaccine on the occurrence of complications were investigated. The percentage of missing data was meager, and given that the side effects were evaluated among the healthcare workers, more accurate answers were obtained.
Literature has proved that vaccination side effects may originate from vaccines themselves. Yet, we have to consider healthcare workers were the first group of society vaccinated and as vaccination pioneers, may experience inefficiency in the vaccine supply chain and distribution system. Therefore, vaccination errors might have affected the outcome. Further evaluation of the vaccinated population and comparison with older results may help to distinguish occurred errors in initial systems.
Conclusion
Our results showed the highest and the lowest occurrence rate of side effects for AstraZeneca and Sinopharm vaccines, respectively. The need to refer to medical centers after vaccination with AstraZeneca was more than that for Sinopharm. The side effects of inactivated virus vaccines such as Sinopharm and Covaxin were lower than that of viral vector vaccines. The negative impact of the Sinopharm vaccine on the ability to do daily routines was lower than other vaccines, and AstraZeneca had the highest effect. There was no significant relationship between a previous history of allergy to the influenza vaccine and the occurrence of side effects. However, such a relationship was observed for the Sinopharm vaccine. There was no significant relationship between BMI and the event of side effects. There was no significant relationship between age and side effects. However, the occurrence rate was significantly higher among younger healthcare workers vaccinated with Sinopharm. There was no significant relationship between a previous history of COVID-19 and the frequency of side effects among those receiving Sputnik, Covaxin, and AstraZeneca vaccines; however, it was significantly higher among those receiving Sinopharm vaccine with a previous history of COVID-19 disease. There was no significant relationship between the history of COVID-19 and referral to medical centers. The most likely time for the occurrence of complications was the first 24 hours, as the probability of the occurrence of side effects on the second day was one-fourth of the first day for AstraZeneca, Covaxin, and Sinopharm and about one-seventh of the first day for Sputnik vaccine and then decreased significantly. Fever, fatigue, and muscle pain were the most common post-vaccination side effects. In order to confirm these findings, it is suggested to re-examine the results in more extensive clinical trials, especially for Covaxin and Sputnik, for whom a smaller statistical population was investigated.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
Researchers are sincerely grateful to the Ethics and Research Committee of Aja University of Medical Sciences.
references
- 1. Teixeira da Silva JA. Mandatory COVID-19 vaccines versus personal freedoms: an imperfect balance. Oman Med J 2022 Jul;37(4):e378.
- 2. Al-Jahdhami I, Al-Naamani K, Al-Mawali A, Bennji SM. Respiratory complications after COVID-19. Oman Med J 2022 Jan;37(1):e343.
- 3. Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020 May - Jun;14(3):247-250.
- 4. Hachim IY, Hachim MY, Naeem KB, Hannawi H, Al Salmi I, Al-Zakwani I, et al. Kidney dysfunction among COVID-19 patients in the United Arab Emirates. Oman Med J 2021 Feb;36(1):e221.
- 5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020 Apr;11(7):995-998.
- 6. Al Awaidy ST, Khamis F. Preparing the community for a vaccine against COVID-19. Oman Med J 2020 Oct;35(6):e193.
- 7. Mathioudakis AG, Ghrew M, Ustianowski A, Ahmad S, Borrow R, Papavasileiou LP, et al. Self-reported real-world safety and reactogenicity of covid-19 vaccines: a vaccine recipient survey. Life (Basel) 2021 Mar;11(3):249.
- 8. Nagy A, Alhatlani B. An overview of current COVID-19 vaccine platforms. Comput Struct Biotechnol J 2021;19:2508-2517.
- 9. Bernal JL, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373.
- 10. Harapan H, Anwar S, Yufika A, Sharun K, Gachabayov M, Fahriani M, et al. Vaccine hesitancy among communities in ten countries in Asia, Africa, and South America during the COVID-19 pandemic. Pathog Glob Health 2022 Jun;116(4):236-243.
- 11. Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med 2021 Apr;10(7):1428.
- 12. Al Awaidy ST, Khatiwada M, Castillo S, Al Siyabi H, Al Siyabi A, Al Mukhaini S, et al. Knowledge, attitude, and acceptability of COVID-19 vaccine in Oman: a cross-sectional study. Oman Med J 2022 May;37(3):e380.
- 13. Asadi Faezi N, Gholizadeh P, Sanogo M, Oumarou A, Mohamed MN, Cissoko Y, et al. Peoples’ attitude toward COVID-19 vaccine, acceptance, and social trust among African and Middle East countries. Health Promot Perspect 2021 May;11(2):171-178.
- 14. Tavakoli N, Nafissi N, Shokri S, Fallahpour M, Soleimani S, Riahi T, et al. Pediatric and adolescent COVID-19 vaccination side effects: a retrospective cohort study of the Iranian teenage group in 2021. J Med Virol 2022 Oct;94(10):4890-4900.
- 15. Zare H, Rezapour H, Mahmoodzadeh S, Fereidouni M. Prevalence of COVID-19 vaccines (Sputnik V, AZD-1222, and Covaxin) side effects among healthcare workers in Birjand city, Iran. International Immunopharmacology 2021;101(Pt B):108351.
- 16. Ndwandwe D, Wiysonge CS. COVID-19 vaccines. Curr Opin Immunol 2021 Aug;71:111-116.
- 17. Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, et al. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines (Basel) 2021 Jun;9(6):577.
- 18. Jayadevan R, Shenoy RS, Anithadevi T. Survey of symptoms following COVID-19 vaccination in India. medRxiv 2021.
- 19. Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021 Jul;78(1):142-145.
- 20. National Institute for Communicable Disease. Division of National Health Laboratory Service. [cited 2022 April 14]. COVID-19 vaccine side-effects. Available from: https://www.nicd.ac.za/covid-19-vaccine-side-effects-faq/.
- 21. World Health Organization. Side effects of COVID-19 vaccines. 2021 [cited 2022 April 14]. Available from: https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines.
- 22.CCenters for Disease Control and Prevention. Possible side effects after getting a COVID-19 vaccine. [cited 2022 April 14]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html.
- 23. Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab Syndr 2021 Sep-Oct;15(5):102207.
- 24. Solomon Y, Eshete T, Mekasha B, Assefa W. COVID-19 vaccine: side effects after the first dose of the Oxford AstraZeneca vaccine among health professionals in low-income country: Ethiopia. J Multidiscip Healthc 2021 Sep;14:2577-2585.
- 25. Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt H-P, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel) 2021 Aug;10(8):752.
- 26. Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis 2021 Oct;111:219-226.
- 27. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis 2021 Jul;21(7):939-949.
- 28. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021 Jan;397(10269):99-111.
- 29. Athavale AV. The covid-19 vaccine. Journal of Advanced Research in Medical Science & Technology (ISSN: 2394-6539) 2021;8(1):29-35.
- 30. Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci 2021 Jun;25(12):4418-4421.
- 31. Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, et al. Prevalence and risk factors of CoronaVac side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med 2021 Jun;10(12):2629.
- 32. Dawood AA. The Russian vaccine of COVID-19 (Sputnik V) has shown side effects on the volunteers. Asian Journal of Research in Pharmaceutical Sciences 2021;11(3):193-194.
- 33. Jarynowski A, Semenov A, Kaminski M, Belik V. Mild adverse events of sputnik V vaccine extracted from Russian language telegram posts via BERT deep learning model. medRxiv 2021.
- 34. Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, et al. Safety of COVID-19 vaccines. J Med Virol 2021 Dec;93(12):6588-6594.
- 35. Kircheis R. Coagulopathies after vaccination against SARS-CoV-2 May be derived from a combined effect of SARS-CoV-2 spike protein and adenovirus vector-triggered signaling pathways. Int J Mol Sci 2021 Oct;22(19):10791.
- 36. Nogrady B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature 2021 Jul;595(7867):339-340.
- 37. Cattaneo M. Thrombosis with thrombocytopenia syndrome associated with viral vector COVID-19 vaccines. Eur J Intern Med 2021 Jul;89:22-24.
- 38. Wise J. Covid-19: rare immune response may cause clots after AstraZeneca vaccine, say researchers. British Medical Journal Publishing Group; 2021.
- 39. Sørvoll IH, Horvei KD, Ernstsen SL, Laegreid IJ, Lund S, Grønli RH, et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost 2021 Jul;19(7):1813-1818.
- 40. Kadali RA, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 2021 May;106:376-381.