Myelodysplastic syndromes (MDS) are a heterogeneous group of myeloid stem cell disorders characterized by peripheral cytopenias, ineffective hematopoiesis with dysplasia, and an increased risk of transformation to acute myeloid leukemia (AML).1 Prevalence increases with age; the reported prevalence ranges from 7–35 cases per 105 individuals in the western population.1 Males are more frequently affected than females.2
MDS is usually suspected by the presence of refractory cytopenias on a routine analysis of peripheral blood. The diagnosis is based on morphological evidence of single or multilineage dysplasia (MLD) on examination of the bone marrow aspirate and biopsy, in a background of peripheral blood cytopenias, which is not attributable to any other secondary causes clinically.
World Health Organization (WHO) classifies MDS into several distinct subtypes, based on the number of cytopenias, the number of hematopoietic lineages exhibiting significant dysplasia, the presence of ring sideroblast, and the blast percentage in the peripheral blood, bone marrow, and cytogenetics.3 The bone marrow blast count and cytogenetic analysis additionally helps in assessing risk and selecting therapy. Patients with MDS can be stratified according to several internationally accepted scoring systems, the most common ones being the original International Prognostic Scoring System (IPSS) and revised IPSS (IPSS-R).4,5
Data from the Indian subcontinent is limited and shows a disease biology distinct from that of the Western population. This may be attributable to various social and financial issues in India associated with the complete workup, treatment, and follow-up of these patients.
The objective of the study was to assess the clinicopathologic profile of MDS patients, classify them according to WHO classification system, categorize them into IPSS and IPSS-R prognostic subgroups, and evaluate the treatment outcome. The data was also compared with that from existing Western and Indian publications.
Methods
A retrospective, cross-sectional study conducted in the department of pathology, department of clinical hematology, and hemato-oncology of a tertiary care teaching hospital in South India included all patients diagnosed with MDS from January 2017 to December 2019. However, patients under the age of 18, those having overlapping features with myeloproliferative neoplasms, MDS/myeloproliferative neoplasms, those with therapy-related MDS and dyspoiesis secondary to other causes were excluded from the study.
Relevant clinical information was obtained from the electronic medical records of the patient. The clinical details included age, gender, presenting symptoms, and the presence of lymphadenopathy or hepatosplenomegaly. The complete blood counts including hemoglobin (Hb), total white cell count with differentials, and platelet count at the time of diagnosis were evaluated with serum LDH level. The percentage of blasts in the peripheral blood, bone marrow, and dysplasia involving different lineages were also assessed. Histological examination of bone marrow was done in all cases and included reticulin stain for marrow fibrosis and immunohistochemistry for assessment of immature precursors. Results of cytogenetic studies including conventional karyotyping and fluorescence in situ hybridization (FISH) were also obtained. The patients were classified based on the revised 2017 WHO classification system and grouped using IPSS and IPSS-R scoring systems. They were then followed-up over a period ranging from six to 42 months.
The data were entered into Microsoft Office Excel spreadsheets and the variables were analyzed using standard analytic techniques with SPSS (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.). The quantitative variables were expressed as mean and median and qualitative variables were expressed as percentages and proportions.This study was approved by Rajagri Hospital Institutional Ethics Committee with reference number RAJH/2020/016.
Results
Forty-eight patients were diagnosed with MDS during the study period [Table 1]. The incidence of MDS was slightly higher in females compared to males (male to female ratio 1:1.2). Their age ranged from 23 to 91 years with the highest number of patients in the seventh decade of life (mean = 62.1 ±25.9 years, median = 64 years). Distribution of cases according to the age groups and gender are given in Figure 1.All the patients who presented at < 50 years of age were females. The mean age was therefore lower in females (57.5 years, median age = 59.5 years) than males (67.7 years, median age = 66.5 years).The most common presenting complaint was fatigue (39.6%). Eight of the 48 (16.7%) patients had organomegaly at the time of presentation. Of these, five presented with isolated splenomegaly, one with isolated hepatomegaly, and two with hepatosplenomegaly.
Table 1: Clinico-hematological and cytogenetic profile of patients.
|
Age, > 60 years
|
31
|
|
Sex, male
|
22
|
|
Hemoglobin level, < 10 g/dL
|
35
|
|
Absolute neutrophil count, < 1.8 × 109/L
|
27
|
|
Platelet count, < 100 × 109/L
|
19
|
|
WHO 2017 classification
|
|
|
MDS single lineage dysplasia
|
1
|
|
MDS with ring sideroblast
|
3
|
|
MDS with multilineage dysplasia
|
30
|
|
MDS with isolated del (5q)
|
3
|
|
MDS excess blast 1
|
5
|
|
MDS excess blast 2
|
6
|
|
MDS unclassifiable
|
0
|
|
IPSS cytogenetic categories*
|
|
|
Good
|
37
|
|
Intermediate
|
1
|
|
Poor
|
6
|
|
IPSS-R cytogenetic categories*
|
|
Very good
|
0
|
|
Good
|
37
|
|
Intermediate
|
4
|
|
Poor
|
1
|
|
Very poor
|
2
|
|
Risk distribution
|
|
|
IPSS
|
|
|
Low
|
18
|
|
Intermediate 1
|
16
|
|
Intermediate 2
|
8
|
|
High
|
2
|
|
IPSS-R
|
|
|
Very low
|
8
|
|
Low
|
18
|
|
Intermediate
|
9
|
|
High
|
6
|
|
Very high
|
3
|
|
Treatment (n = 34)
|
|
|
Support care alone
|
8
|
|
ESA
|
4
|
|
Hypomethylating agents
|
10
|
|
Immunomodulatory agents
|
4
|
|
Observation
|
8
|
WHO: World Health Organization; MDS: myelodysplastic syndromes; del(5q): deletion 5q; IPSS: International Prognostic Scoring System; IPSS-R: Revised International Prognostic Scoring System; ESA: Erythropoietin stimulating agents.*Cytogenetics report– not available in four patients.
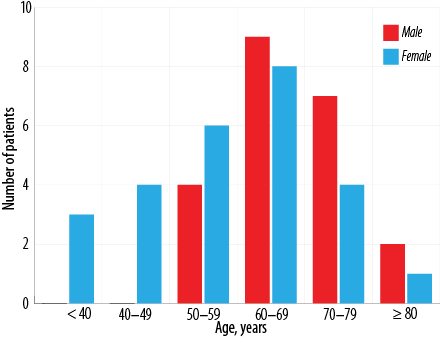 Figure 1: Distribution of myelodysplastic syndromes according to age groups and gender.
Figure 1: Distribution of myelodysplastic syndromes according to age groups and gender.
Routine blood counts revealed variable degrees of cytopenia in most of the patients, with anemia being the most common finding seen in 35 (72.9%) patients (mean Hb = 8.93 g/dL). Of these, 13 patients presented with severe anemia (Hb < 8 g/dL). Pancytopenia was identified in 22.9% and bicytopenia in 27.1% of patients. Only 6.3% of patients had isolated thrombocytopenia (< 100 × 109/L), while isolated leukopenia (ANC < 1.8 × 109/L) and isolated anemia (< 10 g/dL) were seen in 12.5% and 27.1% of patients, respectively. There was no significant difference in the clinical presentation and hematological profile amongst males and females. In addition to varying degrees of dyspoiesis, the peripheral blood smear evaluation revealed the presence of blasts in 18 (37.5%) patients. Serum lactate dehydrogenase (LDH) levels were high in about 50% of the patients (mean serum LDH = 282.6 U/L).
A diagnosis of MDS was made based on the bone marrow examination findings in correlation with clinical details. Dyspoiesis was a key finding in bone marrow aspirate smears, with dyserythropoiesis seen in almost all cases [Figure 2a and 2b]. The high proportion of dyserythropoiesis correlates and thus explains the predominant clinical presentation of fatigue and anemia in the study population. A significant number of ringed sideroblast were seen in three patients, all of whom had associated MLD. Additionally, bone marrow biopsy with immunohistochemistry for CD34 was done for all 48 cases [Figure 2c]. Marrow hypoplasia was seen in eight (16.7%) cases. Increased marrow fibrosis (grades 2 and 3) was also seen in eight patients. The presence of marrow fibrosis was found to have no relation to organomegaly, morphologic subtype, or prognostic risk categories by chi-square test (p < 0.010).
Eleven patients were found to have an increased number of blasts; five patients were subtyped as MDS with excess blasts-1 (MDS-EB-1) and the other six as MDS with excess blasts-2 (MDS-EB-2). Most patients however were of the MDS-MLD subtype (30 patients, 62.5%).
Cytogenetic studies were carried out on 45 patients. In one instance, the results were inconclusive due to insufficient sample material. Cytogenetic abnormalities were noted in 13 (29.5%) of the 44 patients [Table 2]. MDS with deletion 5q [del(5q)] was identified in three cases, all of whom were females that presented with variable degrees of cytopenias but had normal platelets. Bone marrow examination in these patients revealed megakaryocytes with non-lobated and hypolobated nuclei, as described in the literature [Figure 2d].3
Table 2: Distribution of cytogenetic abnormalities in myelodysplastic syndromes patients.
|
Normal
|
31
|
70.5
|
|
Abnormal
|
|
|
|
del (5q)
|
3
|
6.8
|
|
del(20q)
|
3
|
6.8
|
|
Complex
|
2
|
4.5
|
|
Trisomy 7
|
1
|
2.3
|
|
Monosomy 7
|
1
|
2.3
|
|
Others
|
3
|
6.8
|
del: deletion.
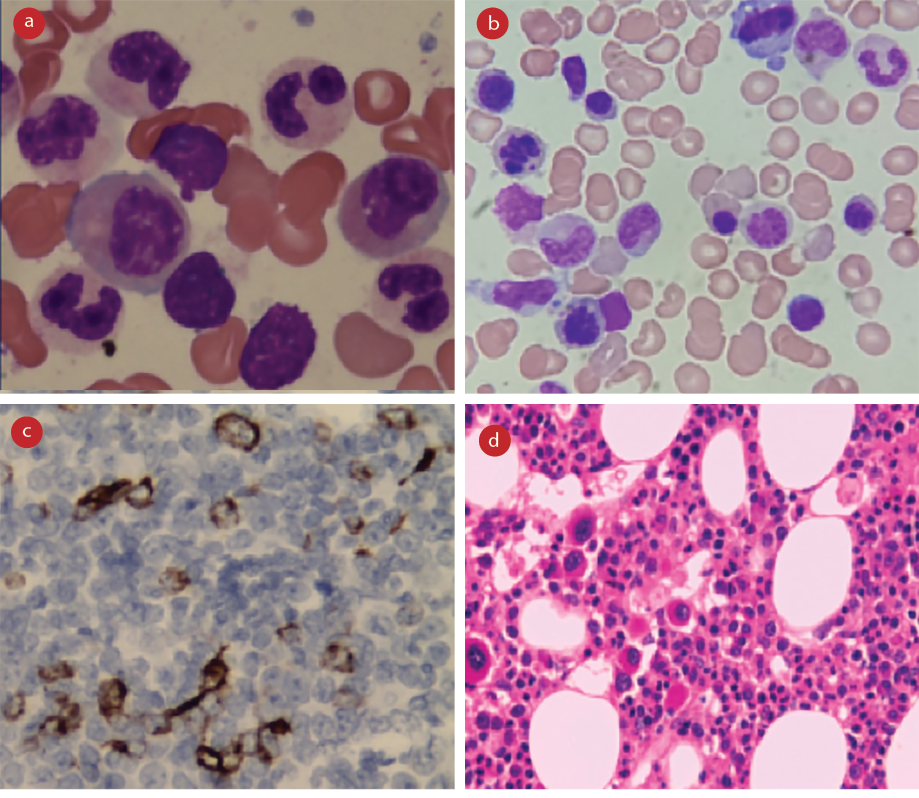 Figure 2: Morphologic features of myelodysplasia. (a) Bone marrow aspiration smear, Leishman stain, magnification = 1000 ×, dysgranulopoiesis. (b) Bone marrow aspiration smear, Leishman stain, magnification = 1000 ×, dyserythropoiesis. (c) Bone marrow biopsy, hemotoxylin and eosin stain, magnification = 100 ×, monolobated megakaryocytes in a case of isolated del(5q). (d) Bone marrow biopsy, CD34 stain, magnification = 400 ×, abnormal localization of immature precursors.
Figure 2: Morphologic features of myelodysplasia. (a) Bone marrow aspiration smear, Leishman stain, magnification = 1000 ×, dysgranulopoiesis. (b) Bone marrow aspiration smear, Leishman stain, magnification = 1000 ×, dyserythropoiesis. (c) Bone marrow biopsy, hemotoxylin and eosin stain, magnification = 100 ×, monolobated megakaryocytes in a case of isolated del(5q). (d) Bone marrow biopsy, CD34 stain, magnification = 400 ×, abnormal localization of immature precursors.
Most patients were noted in the low-risk category (40.9%) when categorized according to the IPSS and IPSS-R scoring systems [Table 1]. Follow-up details were available in 28 patients. The overall mortality of patients with MDS was 57.1% (16/28), 10 of whom were males (62.5%).
Three male patients progressed to AML during the follow-up period. One had MDS-MLD with a cytogenetic abnormality of del(20q), low IPSS-R, and intermediate-1 IPSS category. He was under observation and progressed after three years. Death after disease progression to secondary AML was identified in the other two patients, both of whom had MDS with excess blasts and normal cytogenetic studies at the time of diagnosis. One had MDS-EB-2 with a prognostic category of high IPSS-R and intermediate-2 IPSS; he was started on hypomethylating agent (HMA) but died within one year of initial diagnosis. The other patient had MDS-EB-1 with intermediate and intermediate-1 risk categories according to the IPSS-R and IPSS scoring systems, respectively, was on HMA, and survived for three years before succumbing to the disease.
The patients diagnosed to have MDS with del(5q) were all started on lenalidomide-based chemotherapy. One patient had a mild increase in reticulin at the time of presentation. She progressed over a period of 2.5 years to MDS-EB-1 and eventually succumbed to the disease.
Of the eight patients who presented with increased marrow fibrosis, six (75.0%) died during the follow-up period. The other two were lost to follow-up. Of the 11 patients with MDS with excess blasts, serum LDH levels were raised in seven (63.6%) cases. Serum LDH was raised in 16 out of 37 (43.2%) patients with low-grade MDS.
The 11 patients with MDS with excess blasts were given the option of allogeneic stem cell transplantation. Three of these patients were lost to follow-up while the others were started on HMAs with decitabine/azacytidine. Follow-up marrow assessment was done in five of these patients, two of whom progressed to secondary AML and two showed stable disease. One went into complete remission, received allogeneic stem cell transplant, and is being followed-up. Stem cell transplantation was not done in other cases due to financial reasons or due to donor non-availability.
Discussion
MDS comprises a heterogeneous group of stem cell disorders with respect to the clinical, morphological, and cytogenetic profile. The mean age in this study was 62 years, which contrasts with the lower mean age reported in earlier Indian and Asian studies.6–10 Recent reports from Japan have shown a preponderance in the elderly similar to Western literature.5,11–13 There was a slight female preponderance in our series, unlike other studies that have shown a male predominance. Fifty percent of our female patients were < 60 years of age whereas only 18.2% of the males were < 60 years. This is similar to another study from South India, which showed a younger age incidence in females.14
The most common presenting symptom was fatigue like in other studies. About half of our patients presented with isolated cytopenia of which 27.1% presented with isolated anemia and 6.25% with isolated thrombocytopenia. Only 22.9% of our patients had pancytopenia at the time of presentation and 27.1% had bicytopenia. This contrasts with another Indian study, in which the majority of patients were evaluated for refractory cytopenias, which included pancytopenia in 64% of patients, bicytopenia in 31.3%, and isolated anemia and thrombocytopenia in 3.3% and 1.3% of patients, respectively.15
Interestingly, we found an association between thrombocytopenia and poor outcome (p < 0.010). Of the 28 patients who were followed-up, 10 (35.7%) presented with thrombocytopenia at the time of diagnosis. All these patients died during the course of this study. A study of 2517 patients referred to the MD Anderson Cancer Center with MDS, specifically focusing on the incidence of thrombocytopenia and its association with other prognostic factors and survival outcomes, concluded that thrombocytopenia of any degree was associated with poor survival across all the stages of the IPSS.16
Although identified as one of the cytopenia, the significance of thrombocytopenia at diagnosis or during the disease course is underestimated by the IPSS in which the presence of more than or equal to two cytopenias translates to a score of only 0.5. The IPSS-R though correctly highlights its importance, gives similar weightage to anemia and neutropenia as well.
The incidence of high-grade MDS (MDS-EB-1 and MDS-EB-2) was only 22.9% in our series, which is lower compared to other Indian studies,15 but similar to Western literature.12,13,17
Cytogenetic abnormalities were found in 29.5% of our cases. This is lower than that in other Indian and Western studies.8,9,17 The incidence of isolated del(5q) was 6.8% and complex abnormalities were 5% in our cases, which is also lower than in other reports.9,18
The majority of our patients belonged to the IPSS low category followed by intermediate-1 which together constituted about 77.3% of the cases. In the other study from South India, more cases were noted in the intermediate-1 risk category.14 As per the IPSS-R risk stratification, 59.1% belonged to low and very low-risk categories, and 20.1% to high and very high-risk categories. This is in comparison to the multicentric study which compiled and evaluated 7012 primary untreated MDS patients from multiple international institutions including Spanish, French, Piemonte (Italy), and Brazilian MDS Registries and the International MDS Risk Analysis Workshop. IPSS-R stratification revealed 57% of patients in the very low/low-risk and 23% of patients in the high/very high-risk categories.5 Other Indian studies have shown a larger number of patients in the high and very high-risk categories.14,15,19 This variation in risk profile may be attributable to underdiagnosis of low-grade MDS cases. Many of our patients presented with isolated cytopenias and bicytopenia unlike the other studies, which showed most cases presenting with pancytopenia. A detailed workup of patients presenting with refractory cytopenias may be the reason for the increased detection rate of low-grade MDS in our series.
Cytogenetic abnormalities were detected in a smaller number of cases than expected. This may be because conventional karyotyping alone was done in most cases. Inclusion of FISH may lead to detection of a greater number of cytogenetic abnormalities.
In addition to the risk factors included in the IPSS and IPSS-R categories, the presence of increased marrow fibrosis was strongly associated with poor outcome. Earlier studies evaluating the presence of bone marrow fibrosis in MDS and its clinical relevance, have shown conflicting results due to the heterogeneity of patients included in each series and of the grading systems adopted. Fibrosis was reported in 12–50% of cases, and some authors suggested that the presence of fibrosis may identify a group of patients with a negative prognosis.20 Recent investigations clarified the prevalence and the clinical impact of bone marrow fibrosis in MDS classified according to the WHO criteria. The survival of patients with moderate-to-severe fibrosis is significantly worse than that of patients with no or mild fibrosis, both because of a consequence of profound marrow failure and of the increased rate of leukemic evolution.21,22 At present, MDS with fibrosis is not recognized as a distinctive subtype in the WHO classification.
Hypoplastic MDS (hMDS) is characterized by decreased marrow cellularity, is often difficult to distinguish from aplastic anemia based on standard morphological criteria, and is not currently considered a separate entity by the WHO. Hypocellularity is defined as bone marrow cellularity of < 30% in patients < 70 years or < 20% in those > 70 years. Marrow hypoplasia was identified in 16.7% of our patients. This is comparable with other published data of 8–20%.23,24 All of these patients (except the two with complex cytogenetic abnormalities and high IPSS-R) have shown stable disease. In a large series of 100 hMDS, the rate of progression to AML was lower in hMDS compared to four non hMDS groups (3% vs. 13%, p < 0.020). The median overall survival was longer for patients with hMDS compared with the non hMDS.25 The findings of this study must be seen in the light of some limitations such as small sample size and single-center study.
Conclusion
Our patients were older when compared to those of other Indian studies, with most in the low-risk categories, which is similar to Western data. Females were more commonly affected and presented at a younger age than males, maybe due to the better health-seeking behavior of females. Anemia and fatigue were the most common manifestations of MDS. Most of our patients belonged to the low and very low IPSS-R risk categories similar to data from the west, which is in contrast to other Indian data. Larger population-based studies with follow-up data are required to better understand this regional variation. Cytogenetic testing must include FISH as a part of the complete workup as karyotyping alone may not detect all cytogenetic abnormalities.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med 2009 Nov;361(19):1872-1885.
- 2. Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood 2008 Jul;112(1):45-52.
- 3. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016 May;127(20):2391-2405.
- 4. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997 Mar;89(6):2079-2088.
- 5. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012 Sep;120(12):2454-2465.
- 6. Oguma S, Yoshida Y, Uchino H, Maekawa T, Nomura T, Mizoguchi H; Anemia Study Group of the Ministry of Health and Welfare. Clinical characteristics of Japanese patients with primary myelodysplastic syndromes: a co-operative study based on 838 cases. Leuk Res 1995 Mar;19(3):219-225.
- 7. Kuendgen A, Matsuda A, Germing U. Differences in epidemiology of MDS between Western and Eastern countries: ethnic differences or environmental influence? Leuk Res 2007 Jan;31(1):103-104.
- 8. Vundinti BR, Kerketta L, Jijina F, Ghosh K. Cytogenetic study of myelodysplastic syndrome from India. Indian J Med Res 2009 Aug;130(2):155-159.
- 9. Chaubey R, Sazawal S, Dada R, Mahapatra M, Saxena R. Cytogenetic profile of Indian patients with de novo myelodysplastic syndromes. Indian J Med Res 2011 Oct;134(4):452-457.
- 10. Intragumtornchai T, Prayoonwiwat W, Swasdikul D, Suwanwela N, Chaimongkol B, Jootar S, et al. Myelodysplastic syndromes in Thailand: a retrospective pathologic and clinical analysis of 117 cases. Leuk Res 1998 May;22(5):453-460.
- 11. Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014 Feb;28(2):241-247.
- 12. Haase D, Germing U, Schanz J, Pfeilstöcker M, Nösslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 2007 Dec;110(13):4385-4395.
- 13. Gangat N, Patnaik MM, Begna K, Kourelis T, Al-Kali A, Elliott MA, et al. Primary myelodysplastic syndromes: the Mayo Clinic experience with 1000 patients. InMayo Clinic Proceedings 2015 Dec;90(12):1623-1638.
- 14. D Febe, R Suman. Profile of myelodysplastic syndrome: a study done at tertiary care centre from South India. Int J Recent Trends Sci Technol 2015;15(3):606-609.
- 15. Gupta R, Rahman K, Singh MK, Kumari S, Yadav G, Nityanand S. Clinico-pathological spectrum and novel karyotypic findings in myelodysplastic syndrome: experience of tertiary care center in India. Mediterr J Hematol Infect Dis 2017 Aug;9(1):e2017048.
- 16. Al Ameri A, Jabbour E, Garcia-Manero G, O’Brien S, Faderl S, Ravandi F, et al. Significance of thrombocytopenia in myelodysplastic syndromes: associations and prognostic implications. Clin Lymphoma Myeloma Leuk 2011 Apr;11(2):237-241.
- 17. Kadam P, Dadabhoy K, Bhisey A, Athale U, Nair C, Nair R, et al. Chromosome investigations & clinical outcome in patients with myelodysplastic syndromes. Indian J Med Res 1995 Apr;101:163-169.
- 18. Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol 2008 Jul;87(7):515-526.
- 19. Narayanan S. Clinical, hematological, and cytogenetic profile of adult myelodysplastic syndrome in a tertiary care center. J Blood Med 2017 Feb;8:21-27.
- 20. Lambertenghi-Deliliers G, Orazi A, Luksch R, Annaloro C, Soligo D. Myelodysplastic syndrome with increased marrow fibrosis: a distinct clinico-pathological entity. Br J Haematol 1991 Jun;78(2):161-166.
- 21. Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol 2009 Feb;27(5):754-762.
- 22. Buesche G, Teoman H, Wilczak W, Ganser A, Hecker H, Wilkens L, et al. Marrow fibrosis predicts early fatal marrow failure in patients with myelodysplastic syndromes. Leukemia 2008 Feb;22(2):313-322.
- 23. de Souza DC, Fernandez CdeS, Camargo A, Apa AG, da Costa ES, Bouzas LF, et al. Cytogenetic as an important tool for diagnosis and prognosis for patients with hypocellular primary myelodysplastic syndrome. Biomed Res Int 2014;2014:542395.
- 24. Huang TC, Ko BS, Tang JL, Hsu C, Chen CY, Tsay W, et al. Comparison of hypoplastic myelodysplastic syndrome (MDS) with normo-/hypercellular MDS by International prognostic scoring system, cytogenetic and genetic studies. Leukemia 2008 Mar;22(3):544-550.
- 25. Hunt AA, Khan AB, Potter VT. Hypoplastic MDS is a distinct clinico-pathological entity with somatic mutations frequent in patients with prior aplastic anaemia with favorable clinical outcome. Blood 2014;124(21):3269.