The incidence of end-stage kidney disease (ESKD) has been increasing over the past few years as a direct result of the growing percentages of individuals with metabolic syndrome.1,2 From 2001 to 2015, there were 2805 individuals diagnosed with ESKD in Oman. The majority were caused by diabetic nephropathy (46%), followed by hypertensive nephropathy (19%), and chronic glomerulonephritis (15%).1,2 Genetic or inherited disease formed only 4% of the total cases. More strikingly, most of those were aged between 45 and 64 years old.1,2 The preferred method of renal replacement therapy in Oman is hemodialysis, however, a renal transplant program was established in 1988.1–3 The majority of patients in Oman have commercially transplanted abroad in Pakistan and China, which is criminalized in Omani law.2,4
Without the evolvement of immunosuppressive medications, the dream of organ transplants would not have come true. Since the first light of hope in 1949 when the Nobel Prize winner Philip Hench discovered the anti-inflammatory effects of steroids, the field of immunosuppressive pharmacology has grown.5 In the 1960s, azathioprine was proven to be effective in preventing allograft rejection. Cyclophosphamide was discovered between the 1960s and 1970s, followed by monoclonal antibodies and cyclosporine A between 1970s and 1980s.6,7
By the 1990s, scientists were looking into a much bigger cause of graft rejections; T and B lymphocytes. Thus, antimetabolite medications mycophenolate and leflunomide that target the proliferation of these cell lines came to light.6 In the early 2000s, mycophenolate has become a major cornerstone in immunosuppressive regimens, especially in renal transplant patients. The most common mycophenolate side effects have been reported to affect the hematopoietic and gastrointestinal (GI) system.8
We are reporting an interesting case of mycophenolate-induced colitis with an emphasis on the pathogenesis of colitis caused by mycophenolate and the factors that affect the development of the disease.
Case report
A 42-year-old female, known case of glucose-6-phosphate dehydrogenase deficiency and was diagnosed with hypertension during her last pregnancy (for which she was started on medications). She was later known to have ESKD secondary to chronic glomerulonephritis. She underwent a renal transplant on 1 July 2019 (living-related kidney transplant from her brother) followed by immunosuppressive therapy with methylprednisolone (MP) and basiliximab. In September 2019, she was treated for latent TB (confirmed with a positive Quantiferon test) with nine months course of isoniazid and pyridoxine. The patient was discharged home on tacrolimus 3 mg BID, mycophenolate mofetil (MMF) 1000 mg BID, and prednisolone 15 mg BID as immunosuppressive regimen post-transplant, which was started in July 2019.
On 29 November 2020, she was referred from a renal unit at her regional secondary hospital for evaluation of derangement in the graft function and proteinuria. During admission, the patient reported having longstanding non-bloody watery diarrhea for more than three months and that she was passing more than 10 motions per day. It was not associated with abdominal pain or fever. She reported no associated nausea or vomiting or other GI symptoms. A review of other systems was unremarkable. Her general and systemic examinations during the admission were within normal ranges except for mild bilateral pedal edema.
Initial workup showed no raised inflammatory markers, her white blood count count was 5.6 × 109/ L (normal range = 2.4–9.5), absolute neutrophil count of 4.5 × 109/L (normal range = 1–4.8), lymphocytes 0.7 × 109/L (normal range = 1.2–3.8), eosinophils of 0 × 109/L, and C-reactive protein of < 4.0 mg/L [Table 1]. Erythrocyte sedimentation rate was < 1. Tumor markers: cancer antigen (CA) 15-3 in serum was 25 U/ mL (normal range = 0–33), (CA 19-9 in serum was 9 U/m L (normal range = 0–31), while CA-125 in serum was 85 U/m L (normal range = 0–35). Assessment of the renal function showed creatinine of 122 µmol/L, urea of 5.0 mmol/L, and estimated glomerular filtration rate of 45 mL/min/1.73m2. Urinary proteins were 1.2 g/L. Other biochemical testing showed albumin of 25 g/L (normal range = 34–50). Fecal microscopy sent during admission showed loose stool consistency, no parasites, ova, or cysts as well as no white or red blood cells. Stool culture showed no bacterial growth. Clostridium difficile testing came negative for toxins A, B, and glutamate dehydrogenase. Cytomegalovirus (CMV) level was lower than 100 copies (below the detection level of the laboratory) and testing for BK polyoma virus was negative. Abdomen ultrasound showed no abnormalities except for mild free peritoneal fluids. The patient underwent colonoscopy, which showed normal-appearing mucosa and no abnormalities. However, multiple biopsies were taken throughout the colon. In addition, the patient underwent an allograft renal biopsy as a part of the evaluation for the derangement in renal function.
Table 1: Patient's laboratory test results.
|
Complete blood count
|
|
|
|
|
Hemoglobin
|
10.3 g/dL
|
9.6 g/dL
|
9.4 g/dL
|
|
Platelets
|
200 × 109/L
|
193 × 109/L
|
155 × 109/L
|
|
Total WBC
|
5.6 × 109/L
|
5.1 × 109/L
|
5.8 × 109/L
|
|
Neutrophils
|
4.5 × 109/L
|
4.5 × 109/L
|
4.3 × 109/L
|
|
Eosinophils
|
0 × 109/L
|
0 × 109/L
|
0 × 109/L
|
|
C-reactive proteins
|
< 4.0 mg/L
|
NA
|
NA
|
|
Renal function test
|
|
|
|
|
Creatinine
|
122 μmol/L
|
122 μmol/L
|
104 μmol/L
|
|
Urea
|
5.0 mmol/L
|
7.4 mmol/L
|
12.3 mmol/L
|
|
eGFR
|
45 mL/min/1.73 m2
|
45 mL/min/1.73 m2
|
54 mL/min/1.73 m2
|
|
Urine protein creatinine ratio
|
|
|
|
|
Total proteins
|
1.20 g/L
|
NA
|
NA
|
|
Ratio
|
406.1 mg/umol
|
NA
|
NA
|
WBC: white blood count; eGFR: estimated glomerular filtration rate; NA: not applicable.
She was discharged on 3 December 2020 with a close follow-up appointment to trace the histopathology reports of both renal and colonic biopsies, during which she continued the same immunosuppressive regime. The allograft kidney biopsy was reported with no evidence of active rejection, no glomerular pathology by light microscopy, and only immunoglobulin M deposits of unknown significance were noted on immunofluorescence. Immunohistochemistry testing was negative for C4d and CMV. The colonic biopsies taken from the sigmoid colon showed mildly increased crypt apoptosis, mild architectural disarray, and focal crypt attenuation. This was accompanied by a mild increase in the subepithelial collagen band with focal surface epithelial sloughing [Figure 1]. MMF was stopped and replaced with everolimus with close follow-up appointments to check the progression of the graft derangement and any diarrhea. On subsequent visits, the patient reported complete resolution of diarrhea and further testing showed relatively stable graft function.
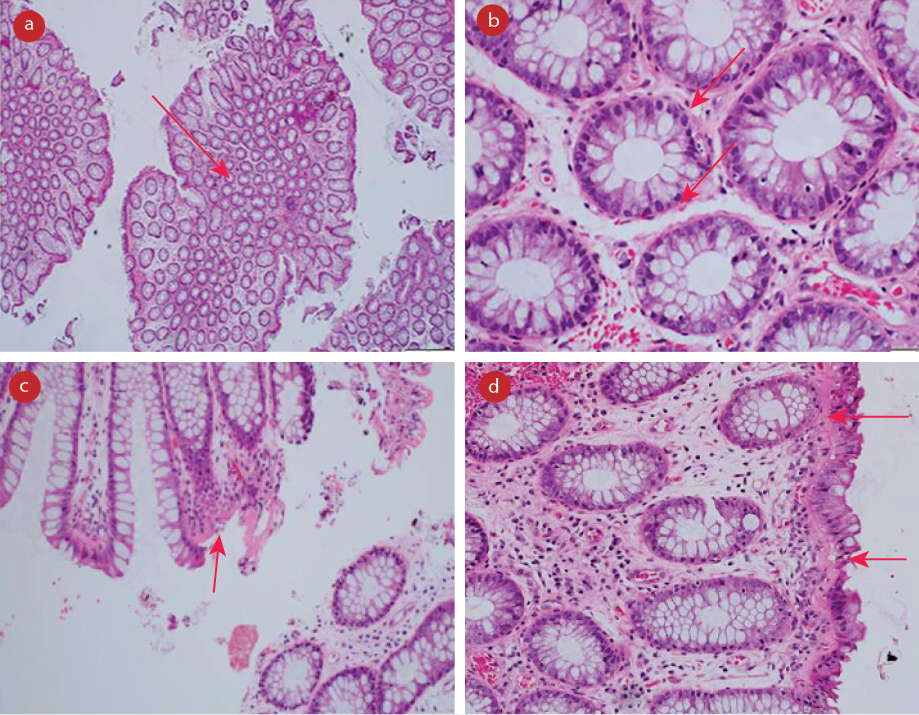 Figure 1: Colonic biopsies taken from the sigmoid colon. It showed a mild increased crypt apoptosis, mild architectural disarray, and focal crypt attenuation. This was accompanied by a mild increase in the subepithelial collagen band with focal surface epithelial sloughing. (a) Preserved architecture, magnification = 2 ×. (b) Basal apoptosis, magnification = 40 ×. (c) Thickened subepithelial collagen, magnification = 20 ×.
Figure 1: Colonic biopsies taken from the sigmoid colon. It showed a mild increased crypt apoptosis, mild architectural disarray, and focal crypt attenuation. This was accompanied by a mild increase in the subepithelial collagen band with focal surface epithelial sloughing. (a) Preserved architecture, magnification = 2 ×. (b) Basal apoptosis, magnification = 40 ×. (c) Thickened subepithelial collagen, magnification = 20 ×.
(d) Thickened collagen with epithelial separation, magnification = 20 ×.
Discussion
Mycophenolate is a prodrug that was first isolated from the organism Penicillium brevicompactum, which is metabolized by the liver to its active drug formulation mycophenolic acid (MPA). It has been described as having both antibacterial and anti-neoplastic activities.8,9 MPA causes prevention of the de novo synthesis of cells by reversible inhibition of inosine monophosphate dehydrogenase causing derangement in the intracellular pool of the nucleotides leading to an arrest in the proliferation of the T and B lymphocytes, hence causing immunosuppression.8–10
Randomized controlled trials showed that if mycophenolate is used with cyclosporine and steroids causes better prevention of allograft rejection compared to a regimen based on azathioprine with other immunosuppressive medications.11 Thus, since the early 2000s, mycophenolate has been widely used in solid organ transplanted patients.12,13 Two widely available formulations of the drugs used world-widely; MMF,6,8,9,13 which was used in our patient, and the enteric-coated mycophenolate sodium.4 The oral bioavailability of MMF is about 80–94%.14,15 After its absorption, mycophenolate is metabolized by the liver to produce the active drug form, MPA, which will be 97–99% bound to blood albumin. Only the free blood MPA exerts the pharmacological effects of immunosuppression.16 MPA is eliminated predominantly by the renal system; however, a small portion is removed through the gut.17
The side effects of mycophenolate are directly proportionate to the blood concentration of the MPA level.18 In addition, lower concentrations have been responsible for more rejection probability.18 In a sense, mycophenolate is a double-edged sword. In this patient, we used 1000 mg twice daily oral dose. The concentration of MPA in the blood has been reported to be affected by many factors including protein binding factors, enterohepatic circulation interruptions, and a decrease in the hepatic glucuronyl transferase activity that is used in the metabolism of the prodrug, which can be caused using glucocorticoids. The level of serum binding to albumin is affected by the level of serum albumin and the use of aspirin.19,20 In our case patient, serum albumin at presentation was 25 g/L because of proteinuria and the patient was not on aspirin. Other drugs that can increase the level of blood MPA are antivirals like acyclovir and probenecid, which are used for hyperuricemia but were not used for our patient.12,16,18,21,22
Mycophenolate is targeting rapidly growing tissues that depend on purine synthesis. The two major organs dependent on this pathway for regeneration are lymphocytes and gut cells.6,23,24 Lymphocytes (B and T cells) are more dependent on this pathway (90%), hence the immunosuppression.23,25 Thus, patients on MMF are vulnerable to T and B lymphocytes dependent organismal infections such as CMV, candida, herpes viruses, bacterial pneumonia, and to larger extent septicemia.9 However, enterocytes are caught in the fire as they are partially dependent on the pathway.26,27 To elaborate, enterocytes are 50% dependent on the pathways targeted by mycophenolate,26,27 and it is estimated that 45% of patients develop GI side effects,8,13,26–29 such as simple diarrhea, esophagitis, gastroesophageal reflux disease, enteritis, and colitis like our patient.30 GI symptoms of MMF include nausea, vomiting, diarrhea, and abdominal pain mostly in the first six months from the initiation of the therapy.26–28 However, it has been reported that patients on MMF can develop mycophenolate-induced colitis between six months and 15 years after the first use of MMF with an average of three years.31 In our patient, the symptoms started one and a half years after the initiation of the treatment following the transplant.
Although MMF-induced colitis is a recognized disease entity, many of the disease features remain shady and unclear. Thus, no official guidelines were formulated for the management of patients complicated by colitis. The most recognized mechanism of pathogenesis of MMF-induced colitis is the production of acyl glucuronide, a metabolite of MPA that directly promotes the release of cytokines like interleukin-6, tumor necrosis factor -alpha, and messengerRNA as well as it binds to plasma proteins, nucleic acids, and the lipids to form a neoantigen that subsequently triggers the immune system to cause inflammation in the form of colitis by hypersensitivity and autoimmunity pathways.26 Another well-recognized theory is that since MMF has antibacterial properties, it poses damage to the normal gut flora leading to anaerobic organism growth that in turn causes tissue damage and inflammation.24 Furthermore, studies suggested further changes like superimposed infections by CMV, campylobacter, and other bacteria that our workup for the patient excluded. Moreover, impaired mucosal regeneration and increase in the goblet cells with reducing the number of the absorptive cells leads to impaired absorption and diarrhea, which was absent in the histopathological examination of our patient’s colonic biopsies.26
In our case report, we found no initial biochemical evidence of inflammation (e.g., no raised C-reactive protein or raised white blood counts) which did not agree with the previously published report by Moroncini et al.32 However, a review showed that patients with MMF-induced colitis could have a negative initial workup.30 The most common imaging findings of the disease are thick-walled edematous bowel with occasional fat stranding that can be evident by ultrasonic evaluation, computed tomography, or even magnetic resonance enterography.15 In contrast, our patient ultrasonic evaluation showed none of the mentioned findings.
Specific histopathological features of MMF-related colitis include crypt architectural disarray, increased lamina propria inflammation, dilated damaged crypts, increased crypt epithelial apoptosis, and graft-versus-host disease- like changes.33 In our patient, the colonoscopy showed a deceiving normal mucosa compared to what has been shown in the tissue examination. A previous study showed that up to 47% of patients could have normal appearance mucosa in patients with histopathological evidence of MMF-induced colitis.26 A poor correlation between the severity of the colitis by colonoscopy and histological disease activity was also suggested by Papadimitriou et al.34 In contrast, the other portion of patients with MMF colitis reported having erythema with multiple petechiae, erosions, and ulcerations with exudative base and flat margins.35
A study published in 2015 analyzed 36 patients post solid organ transplant that presented between 2000 and 2010 with MMF-associated colitis found that only 9% of patients had colitis diagnosed by colonoscopy.26 The most common presentation was diarrhea in 83% of patients. Half of the patients had a histological feature of acute colitis-like changes, 36% of patients had inflammatory bowel disease-like features, 5.6% had ischemic-like changes, and 8.3% of patients had changes similar to graft-versus-host disease changes.26 However, the classically described changes of the MMF-associated colitis are crypt architecture disarray, dilated damaged crypts, and increased epithelial apoptosis, which was identical to our histopathological findings.30 In further analysis of the previously mentioned study, they found an increased risk of MMF-associated colitis in renal transplant patients compared to other solid organ transplant cases, and the least rate being in patients with liver transplants.26 This can be explained by the fact that the active metabolite of the drug (i.e., MPA) is eliminated by the urinary system, which is affected in a patient with renal transplant.23 Our patient was admitted with deterioration of the graft function and developed symptoms during this period which could have increased the level of the MPA in the blood leading to the complication. Another suggested reason is the fact that renal transplanted patients require more immunosuppressive medications compared to other transplanted solid organs.26 As the drug is metabolized to the active form in the liver, this could explain the lower rates of colitis in this subgroup.
There is no current guideline that draws the roadmap to treat patients with MMF-associated colitis. Different reports used different expert opinion-based practices including the most commonly used approach by solely stopping or lowering MMF doses,30,32 which is similar to our approach. In our patient, the MMF was stopped after confirming the diagnosis by histopathology and the medication was replaced by everolimus. Other reports used other approaches like adding steroids as part of the treatment.11 However, no study has compared the efficacy of different treatment options. In our patient, the symptoms stopped subsequently after stopping the mycophenolate and she did not have further episodes of diarrhea during her follow-up appointments. Other reports used other approaches like adding prednisone and/or infliximab as part of the treatment. Bouhbouh et al,36 used a single infusion of 5 mg/Kg of infliximab after failure of response to MMF discontinuation, and 50 mg of prednisolone intravenous daily for two weeks. However, no study has compared the efficacy of different treatment options. In our case, the cause of the renal derangement was under investigation and no specific reason was found.
Conclusion
MMF-induced enterocolitis is uncommon, and it may be associated with debilitating complications. This case shows a unique presentation of MMF-induced colitis with normal biochemical and imaging findings initially. Data is scarce regarding why some patients have refractory colitis and why some get it shortly after using the medications while others get it late, besides the limited data regarding the management approach with the benefits of oral, intravenous steroids, or biologic therapy with infliximab, and the need for endoscopic reassessment for mucosal recovery and healing. We suggest further evaluation of MMF-associated colitis to formulate an evidence-based method to approach patients with MMF-associated colitis, including the criteria for the colonoscopy, possibly mandating biopsy as a large portion of patients can have normal mucosa by the endoscopy, and evaluating treatment options efficacy.
Disclosure
The authors declared no conflicts of interest. A consent was given by the patient.
references
- 1. Al Alawi I, Al Salmi I, Al Mawali A, Al Maimani Y, Sayer JA. End-stage kidney failure in Oman: an analysis of registry data with an emphasis on congenital and inherited renal diseases. Int J Nephrol 2017;2017:6403985.
- 2. Al Ismaili F, Al Salmi I, Al Maimani Y, Metry AM, Al Marhoobi H, Hola A, et al. Epidemiological transition of end-stage kidney disease in Oman. Kidney Int Rep 2016 Sep;2(1):27-35.
- 3. Al Alawi IH, Al Salmi I, Al Mawali A, Sayer JA. Kidney disease in Oman: a view of the current and future landscapes. Iran J Kidney Dis 2017 Jul;11(4):263-270.
- 4. Al Rahbi F, Al Salmi I. Commercial kidney transplantation: attitude, knowledge, perception, and experience of recipients. Kidney Int Rep 2017 Feb;2(4):626-633.
- 5. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocortical hormone in arthritis: preliminary report. Ann Rheum Dis 1949 Jun;8(2):97-104.
- 6. Wiseman AC. Immunosuppressive medications. Clin J Am Soc Nephrol 2016 Feb;11(2):332-343.
- 7. Starzl TE. History of clinical transplantation. World J Surg 2000 Jul;24(7):759-782.
- 8. Hood KA, Zarembski DG. Mycophenolate mofetil: a unique immunosuppressive agent. American Journal of Health-System Pharmacy 1997;54(3):285-294.
- 9. Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl Infect Dis 2009 Aug;11(4):290-297.
- 10. Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit 1995 Dec;17(6):681-684.
- 11. Srinivas TR, Meier-Kriesche H-U. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clinical Journal of the American Society of Nephrology 2008;3(Suppl 2):S101-S116.
- 12. Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol 2014 Jul;88(7):1351-1389.
- 13. Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology 2000 May;47(2-3):215-245.
- 14. Allison AC, Eugui EM. The design and development of an immunosuppressive drug, mycophenolate mofetil. Springer Semin Immunopathol 1993;14(4):353-380.
- 15. Bullingham R, Monroe S, Nicholls A, Hale M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol 1996 Apr;36(4):315-324.
- 16. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 2007;46(1):13-58.
- 17. Metz DK, Holford N, Kausman JY, Walker A, Cranswick N, Staatz CE, et al. Optimizing mycophenolic acid exposure in kidney transplant recipients: time for target concentration intervention. Transplantation 2019 Oct;103(10):2012-2030.
- 18. Md Dom ZI, Coller JK, Carroll RP, Tuke J, McWhinney BC, Somogyi AA, et al. Mycophenolic acid concentrations in peripheral blood mononuclear cells are associated with the incidence of rejection in renal transplant recipients. Br J Clin Pharmacol 2018 Oct;84(10):2433-2442.
- 19. Jung H-Y, Lee S, Jeon Y, Choi J-Y, Cho J-H, Park S-H, et al. Mycophenolic acid trough concentration and dose are associated with hematologic abnormalities but not rejection in kidney transplant recipients. J Korean Med Sci 2020;35(24):e185.
- 20. Mourad M, Malaise J, Chaib Eddour D, De Meyer M, König J, Schepers R, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem 2001 Jan;47(1):88-94.
- 21. Chakrabarti K, Frame D, Al Abbas M, McCune WJ. The use of mycophenolate mofetil area under the curve. Curr Opin Rheumatol 2021 May;33(3):221-232.
- 22. Jaklič A, Collins CJ, Mrhar A, Sorror ML, Sandmaier BM, Bemer MJ, et al. High prevalence of potential drug interactions affecting mycophenolic acid pharmacokinetics in nonmyeloablative hematopoietic stem cell transplant recipients. Int J Clin Pharmacol Ther 2013 Sep;51(9):711-717.
- 23. Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE. PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics 2014 Jan;24(1):73-79.
- 24. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000 May;47(2-3):85-118.
- 25. Karnell JL, Karnell FG III, Stephens GL, Rajan B, Morehouse C, Li Y, et al. Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J Immunol 2011 Oct;187(7):3603-3612.
- 26. Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J, Bhamidimarri KR. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann Gastroenterol 2015 Jul-Sep;28(3):366-373.
- 27. Maes BD, Dalle I, Geboes K, Oellerich M, Armstrong VW, Evenepoel P, et al. Erosive enterocolitis in mycophenolate mofetil-treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation 2003 Mar;75(5):665-672.
- 28. Davies NM, Grinyó J, Heading R, Maes B, Meier-Kriesche HU, Oellerich M. Gastrointestinal side effects of mycophenolic acid in renal transplant patients: a reappraisal. Nephrol Dial Transplant 2007 Sep;22(9):2440-2448.
- 29. Meier-Kriesche HU, Davies NM, Grinyó J, Heading R, Mamelok R, Wijngaard P, et al. Mycophenolate sodium does not reduce the incidence of GI adverse events compared with mycophenolate mofetil. Am J Transplant 2005 May;5(5):1164, author reply 1165-1166.
- 30. Farooqi R, Kamal A, Burke C. Mycophenolate-induced Colitis: a case report with focused review of literature. Cureus 2020;12(1):e6774.
- 31. Goyal A, Salahuddin M, Govil Y. A unique case of mycophenolate induced colitis after 10 years of use. Case Rep Gastrointest Med 2016;2016:3058407.
- 32. Moroncini G, Benfaremo D, Mandolesi A, Gabrielli A. Mycophenolate mofetil-induced colitis in a patient with systemic sclerosis. BMJ Case Rep 2018 May;2018:bcr-2018-224829.
- 33. Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol 2008 Sep;32(9):1367-1372.
- 34. Papadimitriou JC, Cangro CB, Lustberg A, Khaled A, Nogueira J, Wiland A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol 2003 Oct;11(4):295-302.
- 35. Lee S, de Boer WB, Subramaniam K, Kumarasinghe MP. Pointers and pitfalls of mycophenolate-associated colitis. J Clin Pathol 2013 Jan;66(1):8-11.
- 36. Bouhbouh S, Rookmaaker MB. Rapid resolution of persistent mycophenolate mofetil-induced diarrhoea with a single dose of infliximab. Nephrol Dial Transplant 2010 Oct;25(10):3437-3438.