Cystic echinococcosis (CE), also known as hydatid disease, is a worldwide parasitic zoonosis caused by Echinococcus granulosus that is endemic in Middle East countries with a seroprevalence in humans of 10.7% (1996–2000).1 A human seroprevalence study conducted over four months in 1996 involving Omanis attending outpatient departments (n = 306) showed a low prevalence rate of 0.3%.2
Over a 12-year period (2009–2021), we encountered nine cases of CE at Sultan Qaboos University Hospital as referrals after several visits to other institutions and medical specialties in Oman with delayed diagnoses or misdiagnoses. We would like to emphasize the importance of early referral of such patients to an infectious diseases team.
Case Series
Case one
A 21-year-old man from Ad Dakhiliyah governorate presented with a three-week history of intermittent fever and epigastric pain radiating to the left upper quadrant (LUQ). He was afebrile and had tenderness and fullness in the epigastrium. His white blood cells (WBCs), absolute neutrophil count (ANC), and C-reactive protein (CRP) were normal. An eosinophilia of 1 × 109/L was detected. Abdominal computed tomography (CT) showed a 4 × 6 cm complex septated cystic lesion in the liver adjacent to the gallbladder and duodenum [Figure 1].
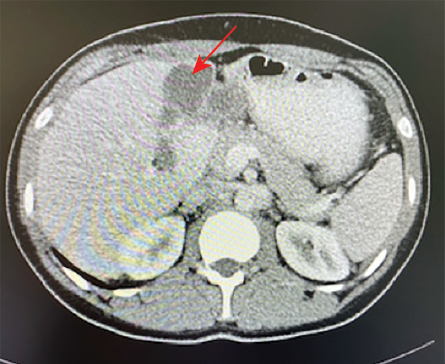 Figure 1: CT abdomen showing a complex cystic lesion in the left hepatic lobe with septations (red arrow).
Figure 1: CT abdomen showing a complex cystic lesion in the left hepatic lobe with septations (red arrow).
The cyst was managed as a pyogenic liver abscess with drainage and antibiotics. Six years later, his symptoms recurred with tenderness in the LUQ. His WBCs and eosinophils were normal and he had high CRP levels of 59 mg/L that dropped to 10 mg/L after three days of antibiotic treatment.
Abdominal magnetic resonance imaging (MRI)showed a 7 × 5.7 cm, well-defined, septated cystic lesion in the previous location. Based on the MRI findings and recurrence of the hepatic lesion, echinococcus serology was requested which was positive by indirect hemagglutination (IHA, Cellognost®, Siemens) at a titer of 1/512. He was started on albendazole followed by a percutaneous puncture, aspiration, injection, re-aspiration (PAIR) procedure and continued for one month with resultant complete resolution of symptoms. Four years later, his symptoms recurred. Abdominal ultrasound (US) showed a 6 × 5 cm cyst in the previous location with adjacent area of inflammation and fibrosis. Albendazole was restarted followed by a laparoscopic cystectomy removing the cyst and all daughter cysts. His serology increased to 1/4096 two months post-PAIR procedure and was 1/1024 during his last presentation.
Case two
A 40-year-old woman from Ad Dakhiliyah governorate presented with a six-month history of right upper quadrant (RUQ) pain and intermittent undocumented fever. She denied animal contact. MRI showed two well-defined, lobulated and non-septated hepatic lesions 13 × 7.7 × 10.8 cm and 4.7 × 3.8 cm in the left hepatic lobe. Echinococcus serology was positive by indirect hemagglutination (IHA) at a titer of 1/4096. Albendazole was started before a PAIR procedure and continued for one month. Aspirated fluid showed protoscolices. In the following two years, she presented twice with an infected cyst growing Pseudomonas aeruginosa necessitating drainage and antibiotics.
Case three
A 21-year-old man presented with a one-month history of left-sided chest pain, productive cough of yellow sputum, intermittent unrecorded fever, and unmeasured weight loss. A left fluid-filled mass was seen on chest X-ray (CXR) and three sputum samples were negative for acid-fast bacill. He was commenced on meropenem and metronidazole for a presumptive diagnosis of lung abscess. After failing to respond to the antibiotic treatment, he was referred to our institution. His WBCs and ANC were normal; he had a CRP of 45 mg/L and eosinophils of 1.6 × 109/L. CT of the chest with contrast showed a left lower lobe abscess with an area of degeneration, an irregular thick wall, and marginal faint enhancement with surrounding consolidation and pleural effusion. He declined bronchoscopy. He received clindamycin and ciprofloxacin for a total of six weeks. Repeated CXR at two months showed a reduction of the mass size with near resolution of his symptoms and eosinophils of 1 × 109/L.
Three years later, he presented with a one-year history of cough productive of yellow sputum and intermittent hemoptysis, associated with left-sided chest pain and a CXR showing a left-sided mass [Figure 2].
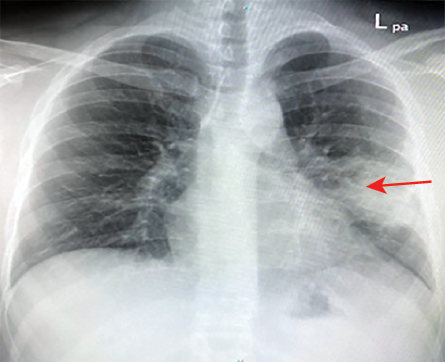 Figures 2: Chest X-ray showing left middle zone mass (red arrow).
Figures 2: Chest X-ray showing left middle zone mass (red arrow).
His WBCs, ANC, CRP, and eosinophils were normal. Chest CT showed a hyperdense mass of 4.6 × 4.9 cm in the same area and bronchoscopy revealed thick whitish secretions mainly in the left lingular bronchus. This was diagnosed as post-abscess cavitary bronchiectasis and he was treated for 14 days with co-amoxiclav followed by elective video-assisted thoracoscopic surgery left upper lobectomy during which whitish fluid was drained. Repeat CXR after 10 days of piperacillin-tazobactam showed full expansion of the lung. Histopathology report mentioned a cavity with degenerative (nematodes)surrounded by fibrosis, thus, he was referred to us (the infectious disease team). Further history revealed his close contact with farm animals. He was started on albendazole 400 mg. Echinococcus serology was requested but unfortunately not done due to reagent unavailability. Screening for liver cysts was negative.
Case four
A 44-year-old man from Dhofar governorate presented with a one-week history of productive cough of large amounts of yellow/brown sputum associated with headache and pain in the chest wall. He denied having fever or hemoptysis.
His chest CT showed an emphysematous thin-walled fluid-containing cyst measuring 4 × 6 cm. This was diagnosed as lung abscess caused by Klebsiella pneumoniae that grew from the sputum and the patient was treated with intravenous antibiotic for two weeks followed by 10 days of oral antibiotics. Due to the persistence of the symptoms and of the cyst on repeat CT, he was referred to our institute. His WBCs and ANC were normal and CRP < 1 mg/L but had elevated eosinophils of 1 × 109/L. Three sputum samples were negative for acid-fast bacilli . His CT showed a pulmonary left upper lobe unilocular, 6.7 × 5 × 5 cm cyst with no enhancement and multiple unilocular cysts in the right liver lobe of the liver suggestive of hydatid cysts [Figure 3].
 Figure 3: (a) Cyst in the middle zone of left lung. (b) Multiple cysts in the liver (red arrows).
Figure 3: (a) Cyst in the middle zone of left lung. (b) Multiple cysts in the liver (red arrows).
Echinococcus serology was positive by IHA (no titer provided). The patient declined cystectomy. He was discharged on 400 mg of albendazole for three months. Six months later, CT showed a smaller lung cyst of 4.8 × 4.6 cm containing air and membranes but no fluid and slightly smaller liver cysts. One month prior to this CT he had a severe bout of cough expectorating a large volume of copious sputum and white liquid suggesting cyst rupture.
Due to his declining cystectomy, albendazole was prescribed for one year, which he took only for three months. Echinococcus serology was not done twice due to unavailability of the kit and was 1/256 and 1/32 in the following six months.
Case five
A 78-year-old man from Ad Dakhiliyah governorate presented with a two-week history of RUQ pain and fever. He had high inflammatory markers (WBCs = 17 × 109/L, ANC = 14.3 × 109 /L, CRP = 152 mg/L) with a normal eosinophil count of 0.1× 109/L. His CT abdomen showed a 5 × 4 cm septated heterogeneous mass in the gallbladder fossa suspicious of malignancy [Figure 4].
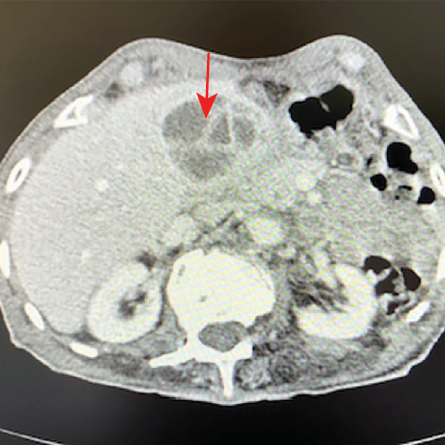 Figure 4: CT abdomen showing septated mass in the gallbladder fossa (red arrow).
Figure 4: CT abdomen showing septated mass in the gallbladder fossa (red arrow).
He underwent US-guided aspiration while on metronidazole and ceftriaxone. The aspirated fluid was mucoid and showed 90% neutrophils and 10% lymphocytes. The bacterial culture was negative. Echinococcus serology was positive at a low titer of 1/64. As this was a low titer and as alveolar echinococcus was in the differential diagnosis (due to radiological features of the cyst), another serum sample was sent to Biomnis-Eurofins laboratories in France. Two serological methods were used (and enzyme-linked immunosorbent assay (ELISA)) to detect immunoglobulin G (IgG) for E. granulosus and E. multilocularis. Results of both tests were negative (hemagglutination of < 160 and IgG by ELISA of 8 KU/L) below the defined reference values. Amoeba serology was also negative.
The patient was treated with albendazole for three months after which a follow-up US abdomen showed near resolution of the hepatic cyst.
Case six
A 31-year-old man (camel caretaker) was referred to our institute for cystectomy of bilateral hydatid cysts in the lungs. He had presented to the local institute with a four-month history of productive cough of yellow sputum, sweating, and 10–15 kg weight loss. Diagnosis of hydatid disease was made based on radiological imaging and positive serology (titer was not provided). He was started on albendazole with a follow-up at five months. Three months later, he represented at the referring hospital with worsening cough, producing salty-tasting white-brown fluid associated with dyspnea, fever, sweating, nausea, and vomiting. A repeat CT demonstrated both cysts to contain septa and daughter cysts and a significant amount of air associated with nodularity and ground glass opacities in the left lung indicating cyst rupture.
At our institute, his WBCs and ANC were normal (CRP = 124 mg/L and eosinophils = 2.9 × 109/L). He was continued on albendazole and echinococcus serology was requested but not done due to unavailability of the kit. Right and left side cystectomies were done six weeks apart. During the interval between the two cystectomies, he developed fever, productive cough, and left-sided chest pain with normal WBCs but raised CRP of 202 mg/L. The team advised stopping albendazole until resection of the left-sided cyst. His sputum grew Haemophilus influenzae and his eosinophils jumped to 2.1 × 109/L two days after left side cystectomy. His last eosinophil count (two months post referral to us) dropped to 1.2 × 109/L.
Case seven
A 71-year-old woman presented to her local hospital with a five-day history of epigastric and LUQ pain. She denied fever, weight loss, or animal contact. CT abdomen showed a 7 × 6 × 6.6 cm hypodense mass with circumferential free-floating membranes [Figure 5] in the left hepatic lobe suggesting a diagnosis of hydatid disease that was supported with positive echinococcus serology by IHA with a low titer of 1/64. She underwent the PAIR procedure and commenced albendazole 400 mg twice daily for three months. The aspirated fluid appeared yellow and turbid containing numerous protoscolices. Her eosinophils were normal throughout except once going up 0.7 × 109/L two days post the PAIR procedure.
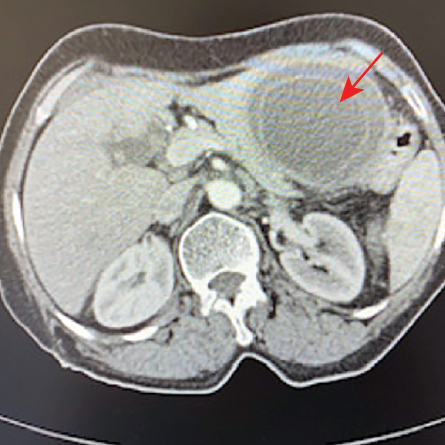 Figure 5: A large mass in the left hepatic lobe with circumferential free-floating membranes (red arrow).
Figure 5: A large mass in the left hepatic lobe with circumferential free-floating membranes (red arrow).
Case eight
A 24-year-old man presented to his local hospital with a 10-month history of exertional dyspnea, wheezing, cough productive of yellow sputum with occasional streaks of blood, intermittent fevers with night sweats, and 13 kg weight loss. Echinococcus serology was positive using ELISA (NovaLisa®). He was started on albendazole a few weeks before referral to our hospital, but he could not tolerate the medication for more than a few days due to gastrointestinal side effects.
His CT chest showed a 5.1 × 4.8 × 5 cm cystic lesion in the right lower lobe and numerous tiny nodules suggestive of cystic rupture. He was referred to the cardiothoracic team at our institute for cystectomy. His WBCs, eosinophils, and CRP were normal. He underwent cystectomy and the aspirated fluid grew P. aeruginosa. He was treated with ciprofloxacin for three weeks and albendazole for one month.
Case nine
A 30-year-old policeman presented to his regional hospital with an eight-month history of intermittent fever with chills, productive cough of yellow sputum mixed occasionally with streaks of blood, anorexia, and undocumented weight loss. A CXR showed a suspicious right-sided hydatid cyst that was supported by a positive serology (the test was done at the central public health laboratory using ELISA). He was started on albendazole 400 mg and discharged with a follow-up appointment scheduled in one month. However, two weeks later he developed a high fever, chest pain, and severe cough for which he was referred for surgical resection of the cyst. Further history revealed regular contact with farm animals.
A CXR showed a 9 × 7.2 cm cyst in the right upper zone and an obscured cardiac silhouette on the left side suspicious of a hidden hydatid cyst that was confirmed with chest CT [Figure 6]. He underwent cystectomy of the right and left sides on consecutive days then started albendazole for one month. A summary of all nine cases is given in Table 1.
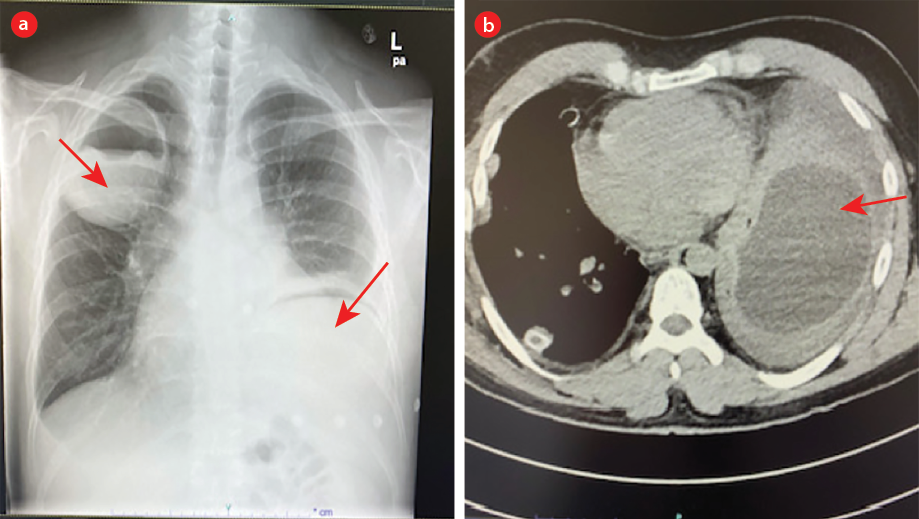 Figure 6: (a) Right upper zone cyst and a suspicious cyst in the left lower zone. (b) CT chest confirms the left lower cyst.
Figure 6: (a) Right upper zone cyst and a suspicious cyst in the left lower zone. (b) CT chest confirms the left lower cyst.
Table 1: Cases of hydatid disease with their presentation, demographic characteristics, and management.
|
1
|
M/21
|
2009
|
3 weeks: epiga-stric pain and intermittent fever
|
Pyogenic liver abscess
|
Liver: L lobe
|
Student
|
Ad Dakhliyah
|
1 × 109
|
3
|
|
Not done
|
Cefuroxime + metronidazole
|
Drainage of abscess
|
|
27
|
2015
Liver
|
RUQ pain,
intermittent
fever, chills,
sweating, and
nausea
|
Hydatid disease
|
Liver: L lobe
|
|
|
Normal
|
59
|
|
IHA: 1/512
|
Ceftriaxone + metronidazole + albendazole days prior to PAIR + 1 month after
|
PAIR
|
|
31
|
2019
Liver
|
Recurrent symptoms
|
Hydatid disease
|
Left
|
|
|
Normal
|
3
|
|
IHA: 1/1024
|
Albendazole
|
Laparoscopic cystectomy
|
|
2
|
F/40
|
2010,
|
6 months of RUQ pain
|
Hydatid disease
|
Liver: L lobe
|
Housewife
|
Ad Dakhiliyah
|
Normal
|
11
|
P. aeruginosa
|
IHA: 1/4096
|
Albendazole × 1 month
|
PAIR
|
|
3
|
M/21
|
2010,
lung
|
1 month of chest pain, SOB, productive cough of yellow sputum, and intermittent fever
|
Lung abscess
|
Left lung
|
Not sought
|
Al Batinah
|
1.6 × 109
|
45
|
|
Not done
|
Meropenem + metronidazole
then 6 wks of clindamycin + ciprofloxacin
|
|
|
24
|
2013
|
1 year productive cough of yellow sputum, hemoptysis, left-sided chest pain
|
|
|
|
|
0.2 × 109
|
Nor-mal
|
|
Not available
|
Co-amoxiclav, then tazocin
albendazole
|
VATS left lobectomy
|
|
4
|
M/45
|
2013
|
1 week of productive cough of large amounts of yellow/brown sputum,
lethargy,
headaches, and
chest wall pain
|
Lung abscess at regional hospital
Then lung and liver hydatid disease
|
Left lung and multip-le hepatic
|
Camel herder
|
Dhofar
|
1
|
< 1
|
K. pneumoniae
|
IHA: 1/256, 1/32
|
Antibiotics
then albendazole (taken 3 months out of 1 year)
|
Declined cystectomy
|
|
5
|
M/76
|
2013
|
3 months RUQ pain and fever
|
Liver abscess then
hydatid disease
|
Liver: L lobe
|
Not
mentioned
|
Ad Dakhiliya
|
Normal
|
152
|
|
Low positive 1/64
|
Ceftriaxone + metronidazole
albendazole × 3 months
|
US guided aspiration
|
|
6
|
M/31
|
2018
|
4 months of fever, sweat-ing, productive cough of yellow sputum, and weight loss
|
Bilateral lung hydatid disease
|
Bilateral lungs
|
Camel caretaker
|
Al Batinah
|
2.9 ×109
|
23
|
H. influenzae
|
Positive. No titer
|
Albendazole × 3 months
|
Bilateral cystectomies 6 weeks apart
|
|
7
|
F/71
|
2020
|
5 days of epigastric and LUQ pain
|
Hydatid disease
|
Liver: L lobe
|
House wife
|
Al Batinah
|
0.3
|
73
|
|
1/64
|
Albendazole × 3 months
|
PAIR. Fluid contained protoscolices
|
|
8
|
M/24
|
2021
|
10 months of productive cough os yellow sputum with streaks of blood, exertional SOB, night sweats, intermittent fever, and weight loss
|
Hydatid disease
|
Lung: R lower lobe
|
Not
mentioned
|
Muscat
|
No
|
4
|
P. aeruginosa
|
Positive at > 11 NTU
|
Albendazole for weeks prior to surgery, then 1 month after surgery
+ 3 weeks ciprofloxacin
|
Cystectomy
|
Ep: eosinophils; RUQ: right upper quadrant; SOB: shortness of breath; L: left; IHA: indirect hemagglutination; PAIR: puncture, aspiration, injection, re-aspiration; VATS:video-assisted thoracoscopic surgery; LUQ: left upper quadrant; R: right; spp: species; NTU; US: ultrasound..
Discussion
CE is a worldwide zoonosis that is particularly endemic in temperate areas including Australia, New Zealand, South Africa, South America, Mediterranean countries, and the Middle East.3 A cross-sectional seroprevalence study conducted on randomly collected sera of various livestock from all governorates of Oman showed the highest positivity in camels (22.4%) followed by cattle (12.9%), sheep (12.2%), and goats (10.9%).4
There is, however, a dearth of information on the prevalence of CE in the human population in Oman. A seroprevalence study conducted on patients attending outpatient clinics in Dhofar in 1996 showed positivity in 0.3% of the 306 patients tested. The sample size was very small and limited to only one governorate of the 11 governorates in Oman, hence could not be representative of the entire population.2 This study was not followed by further similar studies, hence the prevalence of CE in the human population in Oman remains largely unknown.
Transmission of the disease requires the presence of the definitive host involving canid animals and the intermediate hosts, which includes herbivore livestock and humans. The intermediate hosts acquire the infection by consuming food or water contaminated with canids’ feces containing the infective cestode eggs. Humans also acquire the infection through poor hand hygiene following potential contact with canid feces during activities like gardening or contact with infected animals. The eggs hatch in the small intestines of intermediate hosts releasing oncospheres that enter the circulation by penetrating through the wall of small intestines to develop into cysts in various viscera of the incidental hosts.5 The definitive hosts acquire the infection by feeding on animal offal containing the cysts.
Population groups at risk of acquiring the infection include those involved in farming, slaughtering, and dog owners.6
In our study, seven patients were males and two were females. This contrasts with a study from neighboring Iran where prevalence was higher in women possibly due to their greater involvement in the care of livestock.7
Three of our patients reported contact with farm animals, two could not recall any contact, and history was missing for four patients. Transmission of infection in these cases remains unknown. Domestic dogs are rare in Oman on religious grounds; however, stray dogs are not uncommon. Furthermore, food is often ordered from local small restaurants with multiple food handlers. Consumption of food contaminated with dog feces, therefore, remains a possibility.
Liver is the most common site for hydatid cyst development (55%) followed by the lung (28%), but any site can be involved including the brain, skeletal muscle, cardiac muscle, skin, and adrenal.8–11 In our small cohort, four patients (44.4%) presented with liver cysts, four (44.4%) with lung cysts that were bilateral in two patients, and one patient (11.1%) had both lung and liver cysts. This equal proportion of lung and hepatic cysts in our small cohort may in fact be alarming for a possibility of some liver cysts being misdiagnosed as pyogenic liver abscesses. It is essential to screen for liver cysts when patients present with lung cysts. This was very clear in case four whereby the abdominal screening with US revealed multiple liver cysts.
Bilateral cysts occur in 20% of lung cysts,12 yet in our five patients with lung cysts, two patients (40.0%) had bilateral cysts with one patient needing further imaging to identify the cyst behind the left cardiac silhouette.
Post-infection, the disease may remain silent for many years due to the slow growth of the cysts. As the cyst matures and enlarges, it may cause pressure symptoms.13
Clinical manifestations depend on the site and size of the cysts and the occurrence of complications such as rupture of cysts or secondary infections of the cysts. Liver cysts may present with RUQ pains, nausea, and intermittent fevers.13 These symptoms were seen in four of our cases of liver cysts. Sixty-eight percent of hepatic hydatid cysts are reported to occur in the right hepatic lobe, however, four (80%) of our five patients with liver cysts had them in the left lobe.14
None of the cases with liver cysts had jaundice, one had hepatomegaly and one had fullness in the epigastric area rather than hepatomegaly. Jaundice was reported in 9.4% and hepatomegaly in 32.1% of 53 patients from Nepal.15
Lung cysts may present with productive cough of yellow sputum with possible streaks of blood, shortness of breath, weight loss, and intermittent fever that may be associated with sweating mimicking, pulmonary tuberculosis especially with cavitary lesions on chest imaging.16,17 In all of our patients with lung cysts, productive cough with yellow sputum and occasional streaks of blood was a common feature and was associated with pleuritic chest pain.
Fever was intermittent in most of our patients. This agrees with the reported possibility of fever being associated with intermittent leaking of the cyst.18
Complications of hydatid cysts include secondary bacterial or fungal infection and rupture.19,20 In our cohort, case two presented with fever and pain due to relapse of P. aeruginosa infection of residual cyst and case six developed H. influenzae pneumonia. Rupture of the lung cyst may induce severe cough with salty-tasting fluid.21 This was very clearly reported in case four. It may also lead to anaphylactic shock due to the release of a high content of antigen into circulation, activating IgE with the release of histamine.22,23 It may also lead to seeding to other sites and formation of more cysts.24
Leukocyte count may be normal in the majority of patients as was the case with our patients and any rise may be due to secondary infection of the cysts.15 Eosinophilia is not a common finding in hydatid cysts but is a sign of a worse prognosis.25 Perhaps eosinophils increase due to cyst leakage with the fluid antigens triggering immune response associated with intermittent increase in symptoms such as fever, cough, and wheezing.24 In most cases eosinophilia is mild (< 15%) or absent but may increase prominently post-surgery.26 Eosinophilia was present in three of nine patients and increased in three patients post-manipulation.
Liver function tests are mainly normal in hepatic hydatid cysts but may increase with albendazole. In the five patients with liver cysts, liver function tests were normal at presentation.
Diagnosis of CE is established by imaging (plain CXR for lung cysts, abdominal US for hepatic cysts, or CT chest/abdomen) showing a mass lesion that can be found incidentally in case of asymptomatic cysts. Diagnosis is confirmed by serology that needs a high index of suspicion to request it in the first place.27 Abdominal US is the initial imaging modality of choice, which may show characteristic features of hydatid cysts including unilocular and anechoic cysts. Further characterization of these cysts is done with CT scan or MRI, which show characteristic unilocular, simple cyst that may have calcified walls with or without daughter cells.28 On plain CXR, lung cysts appear as well-defined round masses of uniform density that may look like waterlily when endocyst detachment occurs.29 It may also appear as cavitary lesions, which when associated with cough and hemoptysis may mimic tuberculosis.17 In case three, despite the lung fluid-filled cyst seen on plain CXR and initial eosinophilia, serology was not requested as the diagnosis was not considered. For case four, serology was only requested after advice from the infectious diseases team was sought.
Serology is used for diagnosis and follow-up after treatment.30 Several serological methods are available including IHA, ELISA, indirect fluorescent antibodies, and others. Initial screening tests with ELISA or IHA can be confirmed by using highly specific immunoblot or gel diffusion assay. A number of factors can affect the performance of the serological tests including the type of antigen used in the test (e.g., hydatid cyst fluid versus recombinant antigens), cyst size, cyst stage, cyst location (liver cysts having higher sensitivity than lung cysts), and others.31 Cross-reactivity with other parasitic infections can be challenging.32
In our setting, samples received before October 2021 were tested using IHA (Cellognost® Siemens) which detects antibodies against E. granulosus and can provide titers. After October 2021, we moved to use ELISA (NovaLisa®, NovaTec Immunodiagnositca GmbH) which detects IgG against the Echinococcus species. The package insert of the IHA kit clearly states that low positive titers (1/32 to 1/128) should only be accepted as positive when confirmed by another serological method. Upon reviewing all serology results (multiple samples) of our nine cases, we found several obstacles. Some of the reported results did not provide titers because the kit used had quality control issues regarding the titration part. In addition, one sample was tested at another institute that did not provide a titer. Moreover, there were instances when the kit was not available in our laboratory, diagnosis was, therefore, made based on radiological features, previous presentations, and response to treatment.
Although case five had a negative serology, we treated him as hydatid disease based on the typical radiological features of the liver cyst. Follow-up imaging was not possible as the patient defaulted following a cerebrovascular accident.
Current modalities of treatment in general include surgery, percutaneous management, and drug therapy, but specifically depend on the site and the size of the cyst. Hepatic cysts categorized as World Health Organization (WHO) stages CE1 and CE3a (single, < 5 cm in size) or cysts deep in the liver not amenable to percutaneous treatment may be treated with albendazole alone for a duration of one to six months. It is also recommended for patients declining procedures or are not fit for them. PAIR procedure is used for cysts with WHO staging CE1 and CE3a. Another percutaneous treatment is applied on stages CE2a and CE3b that contain daughter cysts or those that relapsed with PAIR.25
PAIR procedure carries a risk of spillage of the cyst contents and so albendazole should be started at least four days before the procedure and continued for one month afterwards.33 Surgery is the treatment choice for complicated cysts (rupture, biliary fistulae, compression on vital structures, secondary infection, or hemorrhage), and big cysts > 10 cm superficial cysts at risk of rupture and for extrahepatic cysts.25
Albendazole is usually started before surgical/percutaneous procedures in case of liver cysts to permit thinning of the cyst wall, reducing its size and killing the larvae. Indeed, with pre-procedure albendazole, viable cysts were reported to be 0–9.37% compared to no albendazole which resulted in viable cysts in 94.5–96.87% at time of surgery. Moreover, recurrence rate was 16.66–18.75% in those not taking albendazole compared to 0–4.16% in those who did.34,35
Cystectomy is the recommended modality for lung cysts with reported morbidity of up to 13% and mortality of up to 5%.36 Albendazole use prior to cystectomy of lung cysts has been reported to trigger cyst rupture by reducing the tensile strength of the cuticular membranes with possible fatal outcome.21,22,37 In all our patients with lung cysts, the use of albendazole was started by non-ID clinicians. It led to rupture of cysts in cases four and eight and possibly nine as evident from the clinical symptoms and supported by images showing signs indicating rupture. Challenges arise when the patient himself decline surgical intervention and albendazole is tried as a treatment in lung hydatid cysts as occurred in case four.
Recurrence of hydatid cysts defined as the appearance of new cysts after therapy was reported to occur in 8.7% of cases.38 In our patients, two patients (22.2%) had a recurrence. Cases one and four had a recurrence six years and five years after diagnosis of CE, respectively. These episodes of recurrence could have been due to inadequate therapy.
Prevention can be achieved by covering food and practicing good hand hygiene. Feeding domestic dogs cooked offal would further aid in disease prevention as liver cysts were shown to be killed by cooking in boiling water for 30 minutes.39
Conclusion
The prevalence of human CE in Oman is unknown. It is one of the WHO’s neglected diseases. This was clearly reflected in the unfamiliarity of the clinicians with the diagnosis and its management including the description of the organism as nematode by the pathologists and the prescribing of albendazole to patients with lung cysts pre-cystectomy. This could be improved by educating medical professionals, publishing encountered cases, and possibly including the disease in the list of notifiable diseases. The present study is, therefore, very important to remind clinicians of this neglected disease and its most common presentations and guide them to the appropriate management.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Galeh TM, Spotin A, Mahami-Oskouei M, Carmena D, Rahimi MT, Barac A, et al. The seroprevalence rate and population genetic structure of human cystic echinococcosis in the Middle East: a systematic review and meta-analysis. Int J Surg 2018 Mar;51:39-48.
- 2. Idris MA, Ruppel A, Gehrig-Feistel H, Alansari AS, al-Rejaibi AK, Tageldin MH, et al. The seroprevalence of cystic hydatidosis in Oman. Ann Trop Med Parasitol 1999 Apr;93(3):259-263.
- 3. Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 2012 Apr;18(13):1425-1437.
- 4. Al-Kitani FA, Mansoor MK, Hussain MH, Al Rawahi AH, Saqib M, Al Maawali MG. Sero-epidemiology of cystic echinococcosis (Echinococcus granulosus) in the livestock of Oman. Vet Parasitol Reg Stud Reports 2017 May;8:21-27.
- 5. Ustianowski AP, Zumla A. Hydatid disease. Clin Microbiol Infect 1998 Jul;4(7):405-409.
- 6. Tamarozzi F, Legnardi M, Fittipaldo A, Drigo M, Cassini R. Epidemiological distribution of Echinococcus granulosus s.l. infection in human and domestic animal hosts in European Mediterranean and Balkan countries: a systematic review. PLoS Negl Trop Dis 2020 Aug 10;14(8):e0008519.
- 7. Moradi M, Rampisheh Z, Roozbehani M, Razmjou E. A retrospective study of hydatid cysts in patients undergoing liver and lung surgery in Tehran, Iran. Heliyon 2019 Jun;5(6):e01897.
- 8. Mahmoudi S, Mamishi S, Banar M, Pourakbari B, Keshavarz H. Epidemiology of echinococcosis in Iran: a systematic review and meta-analysis. BMC Infect Dis 2019 Nov;19(1):929.
- 9. Aleksic-Shihabi A, Vidolin EP. Cystic echinococcosis of the heart and brain: a case report. Acta Med Okayama 2008 Oct;62(5):341-344.
- 10. Özarmagan S, Erbil Y, Barbaros U, Salmaslioglu A, Bozbora A. Primary hydatid disease in the adrenal gland: a case report. Braz J Infect Dis 2006 Oct;10(5):362-363.
- 11. Al-Hakkak SM. Adductor magnus muscle primary hydatid cyst rare unusual site: a case report. Int J Surg Case Rep 2018;51:379-384.
- 12. Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics 2003 Mar-Apr;23(2):475-494, quiz 536-537.
- 13. Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis 2009 Mar;13(2):125-133.
- 14. Alghoury A, El-Hamshary E, Azazy A, Hussein E, Rayan HZ. Hydatid disease in Yemeni patients attending public and private hospitals in Sana’a City, Yemen. Oman Med J 2010 Apr;25(2):88-90.
- 15. Joshi U, Subedi R, Jayswal A, Agrawal V. Clinical characteristics and management of the hydatid cyst of the liver: a study from a tertiary care center in Nepal. J Parasitol Res 2020 Sep;2020:8867744.
- 16. Sheikhy K, Shadmehr MB. Hemoptysis as a complication of capitonnage for management of pulmonary hydatid cyst. Tanaffos 2014;13(3):46-48.
- 17. Lawandi A, Yansouni CP, Libman M, Rubin E, Emil S, Bernard C, et al. A 9-year-old female with a cough and cavitary lung lesion. Clin Infect Dis 2019 Aug;69(4):705-708.
- 18. Gupta N, Vasudevan V, Malik S, Nair N. Disseminated hydatid disease presenting as fever of unknown origin: a case report and review of literature. Med J Dr Patil Univ 2015;8(1):65.
- 19. Koçer NE, Kibar Y, Güldür ME, Deniz H, Bakir K. A retrospective study on the coexistence of hydatid cyst and aspergillosis. Int J Infect Dis 2008 May;12(3):248-251.
- 20. García MB, Lledías JP, Pérez IG, Tirado VV, Pardo LF, Bellvís LM, et al. Primary super-infection of hydatid cyst–clinical setting and microbiology in 37 cases. Am J Trop Med Hyg 2010 Mar;82(3):376-378.
- 21. Sheikhy K, Dezfuli AA, Pejhan S, Beigee FS. Fatal outcome of ruptured pulmonary hydatid cyst. Tanaffos 2018;17(20):138-141.
- 22. Kurkcuoglu IC, Eroglu A, Karaoglanoglu N, Polat P. Complications of albendazole treatment in hydatid disease of lung. Eur J Cardiothorac Surg 2002 Oct;22(4):649-650.
- 23. Sarkar M, Pathania R, Jhobta A, Thakur BR, Chopra R. Cystic pulmonary hydatidosis. Lung India 2016 Mar-Apr;33(2):179-191.
- 24. Derbel F, Mabrouk MB, Hamida MBH, Mazhoud J, Youssef S, Ali AB, et al. Hydatid cysts of the liver - diagnosis, complications and treatment. In: Derbel F, editor. Abdominal surgery. IAbdominal Surgery. 2012 Aug [cited 2022 Mar 11]. Available from: http://www.intechopen.com/books/abdominal-surgery/hydatid-cysts-of-the-liver-diagnosis-complications-and-treatment.
- 25. Botezatu C, Mastalier B, Patrascu T. Hepatic hydatid cyst - diagnose and treatment algorithm. J Med Life 2018 Jul-Sep;11(3):203-209.
- 26. Lv H, Jiang Y, Liu G, Zhang S, Peng X. Surgical treatment of multiple hydatid cysts in the liver of a pediatric patient. Am J Trop Med Hyg 2015 Mar;92(3):595-598.
- 27. Filippou D, Tselepis D, Filippou G, Papadopoulos V. Advances in liver echinococcosis: diagnosis and treatment. Clin Gastroenterol Hepatol 2007 Feb;5(2):152-159.
- 28. Suwan Z. Sonographic findings in hydatid disease of the liver: comparison with other imaging methods. Ann Trop Med Parasitol 1995 Jun;89(3):261-269.
- 29. Santivanez S, Garcia HH. Pulmonary cystic echinococcosis. Curr Opin Pulm Med 2010 May;16(3):257-261.
- 30. Riganò R, Ioppolo S, Ortona E, Margutti P, Profumo E, Ali MD, et al. Long-term serological evaluation of patients with cystic echinococcosis treated with benzimidazole carbamates. Clin Exp Immunol 2002 Sep;129(3):485-492.
- 31. Sarkari B, Rezaei Z. Immunodiagnosis of human hydatid disease: where do we stand? World J Methodol 2015 Dec;5(4):185-195.
- 32. Manzano-Román R, Sánchez-Ovejero C, Hernández-González A, Casulli A, Siles-Lucas M. Serological diagnosis and follow-up of human cystic echinococcosis: a new hope for the future? Biomed Res Int 2015;2015:428205.
- 33. Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010 Apr;114(1):1-16.
- 34. Arif SH, Malik AA, Khaja AR, Dass TA, Naikoo ZA; Shams-Ul-Bari. Role of albendazole in the management of hydatid cyst liver. Saudi J Gastroenterol 2011 Sep-Oct;17(5):343-347.
- 35. Arif SH, Shams-Ul-Bari, Wani NA, Zargar SA, Wani MA, Tabassum R, et al. Albendazole as an adjuvant to the standard surgical management of hydatid cyst liver. Int J Surg 2008 Dec;6(6):448-451.
- 36. Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg 2008 Sep;79(3):301-311.
- 37. Usluer O, Kaya SO, Samancilar O, Ceylan KC, Gursoy S. Thoracic surgery the effect of preoperative albendazole treatment on the cuticular membranes of pulmonary hydatid cysts: should it be administered preoperatively? Pol J Cardio-Thorac Surg 2014;11(1):26-29.
- 38. Prousalidis J, Kosmidis C, Anthimidis G, Kapoutzis K, Karamanlis E, Fachantidis E. Postoperative recurrence of cystic hydatidosis. Can J Surg 2012 Feb;55(1):15-20.
- 39. Li J, Wu C, Wang H, Liu H, Vuitton DA, Wen H, et al. Boiling sheep liver or lung for 30 minutes is necessary and sufficient to kill Echinococcus granulosus protoscoleces in hydatid cysts. Parasite 2014;21:64.