Sleep is a recurrent physiological state of bodily rest and reduced consciousness that serves multiple functions including the consolidation of memory and enhancing the immune defense.1 It is tightly connected to the circadian system that regulates periodic changes in behavioral and physiological parameters. Sleep and the circadian system cooperate bidirectionally to organize immune functions in time and space via neuroendocrine and sympathetic effector mechanisms. Short sleep duration may depress the immune response.2 Furthermore, sleep disturbances such as those occurring in patients with obstructive sleep apnea (OSA) may also cause cellular and humoral immune dysfunctions. COVID-19 infection is associated with elevated levels of inflammatory cytokines and that can be worsened by sleep deprivation and disturbances. Our aim is to explain the interaction between immune mechanism and sleep, and cover the effect of COVID-19 on children and healthcare workers (HCWs).
The circadian clock
Circadian rhythms are essential for a variety of phenomena ranging from microbial growth, plant growth and production, and human wake/sleep cycle. Circadian rhythms have a great influence on human sleep, locomotion, cognition, and feeding and drinking.3 The regulation of circadian rhythms in humans is the function of suprachiasmatic nucleus, which is located in the hypothalamus and receives input from various sensors in the body. At sunset, the signal of the dimming light travels through a neural tract that runs from the retina to the suprachiasmatic nucleus, from which the information is transferred downwards to the preganglionic sympathetic neurons in the spinal cord and onto the superior cervical ganglia upwards to the pineal gland.4 Melatonin, the sleep onset hormone, is then secreted into the bloodstream usually two to three hours after sunset to reach every organ in the body. Circadian rhythms can influence various biological rhythms such as hormone release, blood pressure, body temperature, and metabolism, and lasts approximately 24 hours.5 Furthermore, circadian rhythms act as an internal timekeeping system permitting the cell to predict internal or external changes to perform its cellular activities at suitable times during the day.6
Immune response and circadian rhythm
Immune cells are assigned either to the innate (unspecific; e.g., neutrophils, monocytes, macrophages, dendritic cells and natural killer [NK] cells), or the adaptive immune system (antigen-specific; lymphocytes [T-and B-cells]). In the oscillation of the sleep-wake cycle, leukocytes show robust diurnal rhythms with peak counts at night or during the day, depending on the cell type.7 Leukocytes, such as naive CD4 T cells, monocytes, and neutrophils have nighttime maximum rhythms. These rhythms are regulated by cortisol that leads to an upregulation of the chemokine receptor CXCR4 and thereby facilitates the redistribution of these cells to the bone marrow, with a delay of approximately three hours.8 On the other hand, leukocytes like cytotoxic NK cells have daytime maximum rhythms. These rhythms are regulated by epinephrine which promotes the release of these cells from the marginal pool by an immediate inhibition of adhesive fractalkine receptor signaling.8 Furthermore, pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), interferon gamma, and IL-6 are generally thought to be as somnogenic with increased levels during the rest phase.9 While anti-inflammatory cytokines, e.g., IL-10 and IL-4, have a negative effect on sleep and are high after waking.10
Sleep deprivation and immune response
Several studies indicated that short sleep duration might depress cellular and humoral immunity.11 There are daily rhythms in the changes of IL-1β and TNF-α concentration. Moreover, the expression of the messengerRNA of these molecules in the brain varies with the sleep-wake cycle. The highest levels correlate with high sleep inclination and both IL-1β and TNF-α messenger RNA expression increases in rats’ brains during sleep deprivation.12
Previous studies on the influence of sleep on circulating concentrations of IL-2 and IL-6 had controversial findings. For instance, it has been found that sleep enhances the production of IL-2 by T cells,13,14 while sleep deprivation leads to a decreased IL-2 production from stimulated lymphocytes under the influence of the hypothalamic-pituitary-adrenal axis.15 Moreover, another study showed that five days of sleep deprivation decrease circulating IL-2 and IL-6, which is similar to the effects of acute sleep deprivation and psychological stress.16,17 On the other hand, earlier studies indicated that nocturnal sleep deprivation leads to daytime (post-deprivation period) over-secretion of IL-6 in healthy subjects, and there is a negative correlation between nocturnal sleep and IL-6 release.18 Thus, with good nocturnal sleep and a good sense of well-being, the next day are associated with decreased overall secretion of IL-6.19 Nevertheless, we and others have clearly illustrated that patients with nocturnal sleep disturbance such as obstructive sleep apnea syndrome (OSAS) and pathologically increased daytime sleepiness and fatigue have elevated levels of circulating IL-6, which can activate the hypothalamic-pituitary-adrenal axis and lead to an increased production of cortisol and a depressive mood.11,20,21 OSAS may be associated with inflammatory responses, including the upregulation of IL-1β, IL-6, and pro-Th2 immune responses, increased proliferative potential of NK and CD4 T cells, and a decreased capacity of neutrophils to phagocytose bacteria and produce reactive oxygen species.11 More recently, a study showed that sleep restriction of five hours for one week may lead to decreased phagocytosis and NADPH oxidase activity in neutrophils and a decrease in the levels of CD4+ T cells, which is related to changes in the Th1-related chemokine (CXCL-9 and CXCL-10) balance.22
Impact of sleep deprivation on COVID-19 pandemic
Sleep deprivation is associated with an increased level of inflammatory cytokines including IL-6 and a decrease in CD4 T lymphocyte levels.22–24 COVID-19 infection is associated with elevated levels of inflammatory cytokines including IL-6 and with lymphopenia.25 Therefore, sleep deprivation might worsen these phenomena as it causes similar changes. Moreover, CD4 T cells are crucial in fighting infections including those caused by viruses, therefore their decrease in sleep-deprived patients suggests that sleep-deprived individuals are more susceptible to viral infections.22 In fact, sleep deprivation for seven consecutive days was associated with increased susceptibility to rhinovirus causing common cold. Therefore, it is possible to hypothesize that sleep deprivation increases the susceptibility to SARS-CoV-2 causing COVID-19. Interestingly, a study found that poor sleep quality in hospitalized COVID-19 patients with lymphopenia was associated with a lower absolute lymphocyte count, reduced recovery rate, and an increased requirement for intensive care unit (ICU) care.26 More studies are required to clarify the effect of sleep deprivation on the pathogenesis of COVID-19 infection including its impact on treatments aiming to inhibit the inflammatory responses and the influence of hormones related to sleep such as cortisol and melatonin on COVID-19 pathogenesis, bearing in mind that these hormones have the capacity to decrease the inflammatory responses. Moreover, healthy sleep hygiene that adheres to circadian rhythm would help in fighting COVID-19 symptoms.
Sleep and the immune responses mutual effects during COVID-19
Multiple factors associated with sleep and immune responses might influence the infection with SARS-CoV-2 and are potentially affected by COVID-19. Among these molecules, we can identify IL-6 and melatonin.
The elevated levels of IL-6 during the infection with SARS-CoV-2,27 might associate with the severity of the disease and its mortality.28–31 The treatment of severe COVID-19 cases includes broad anti-inflammatory molecules like dexamethasone and anti-IL-6R drugs28 such as tocilizumab and sarilumab. This reduces the application of mechanical ventilation and mortality in critical and severe cases of COVID-19.32–35 IL-6 can affect the brain by blood-brain-barrier transport and volume diffusion.36 Therefore, it can modulate the different processes implicated in sleep.37 IL-6 has the capacity to bind to its soluble receptor (IL-6Rs) and induce signaling in cells that do not have IL-6R in a process called trans-signaling.38,39 IL-6 induces the majority of its inflammatory effects through trans-signaling.38,39 Although neurons might have low levels of IL-6R,40 IL-6 can potentially affect them through trans-signaling. Interestingly, this trans-signaling is associated with the sleep process.38,39 Different studies demonstrated the presence of association between high levels of IL-6 and other inflammatory markers such as TNF-α and C-reactive protein (CRP) with sleeping difficulties.41–45 IL-6 was suggested to have a strong association with sleeping problems,43 although IL-6 might have somnogenic effects at high levels during the rest phase. Hospitalized COVID-19 patients might develop psychological and neurological disorders.46,47 This might be a direct pathological effect of SARS-CoV-2 itself as the virus might be present in the brain tissues. However, SARS-CoV-2 does not infect neurons.48 This suggests that other factors associated with the pathogenesis of COVID-19 can affect the brain. IL-6 constitutes a potential factor that affects the brain and sleep during severe COVID-19 infections because of its effects on them. This corroborates with the fact that the anti-IL-6R drug tocilizumab abrogates the association of the psychological and neurological disorders in severe cases of COVID-19.49,50 Interestingly, this effect is also observed with anakinra, an anti-IL-1β drug49,50 and the psychological and neurological effects are associated with systemic inflammation occurring in severe COVID-19 infection.49,50
IL-6 might also affect the brain and sleep by an indirect mechanism. The high levels of IL-6 in severe COVID-19 might participate in inducing the differentiation of CD4-T cells to Th17 cells.51,52 This can affect the ratio of Th17 to regulatory CD4 T cells (Tregs).53 The modified Th17/Treg ratio might have an effect on the brain and therefore can potentially affect the sleep process centrally.54
Moreover, sleep itself can regulate the levels of IL-6; however, this effect is controversial.19–22 Some studies reported that sleep deprivation is associated with decreased levels of IL-6,19,20 and that would suggest that lack of sleep during COVID-19 infections might have a decreasing effect on IL-6 levels. However, the concentration of IL-6 in severe COVID-19 reaches high levels,27 and it is unlikely that a decrease in IL-6 levels induced by sleep deprivation might affect these concentrations.29–31 In contrast, the studies indicating the increase of IL-6 levels in sleep-deprived individuals21,22 suggest that sleep deprivation associated with COVID-19 might increase IL-6 levels by installing a circle where the infection with SARS-CoV-2 leads to the increase of IL-6, which can induce sleep deprivation that contributes in increasing IL-6 levels and amplifying the inflammatory process.
Circadian and drugs used for SARS-CoV-2 treatment
Although melatonin is mainly produced by the pineal gland, it is also produced by other cells and organs such as the eyes, gastrointestinal tract, bone marrow, and lymphocytes.55 Melatonin affects the immune responses and both anti-inflammatory and enhancing effects were reported for it.56 Beneficial effects were suggested for melatonin in some viral infections.57 This provides a rationale for the potential therapeutic effect in the infection with SARS-CoV-2. A large study reported that high levels of melatonin were associated with a decreased potential of infection with SARS-CoV-2. This decrease was higher in the African American population (52%) compared to the general population (28%).58
Moreover, beneficial effects were also reported for melatonin in intubated COVID-19 patients.59 Several trials that involve melatonin and its agonists are reported at clinicaltrials.gov. A small study suggested that treatment with 3 mg melatonin for two weeks was associated with rapid hospital discharge, improvement of pulmonary involvement and symptoms including dyspnea, fatigue and cough, and molecules associated with inflammation such as CRP.60 Another pilot study suggested that treatment with 6 mg melatonin for two weeks was associated with higher symptoms recovery.61 In addition, treatment with 3 mg melatonin for one week was associated with higher Leeds sleep evaluation questionnaire scores and oxygen saturation. This study did not detect an association with CRP, white blood cell count, and lymphocyte count.62
Moreover, circadian clocks regulate the pharmacokinetics and efficacy of many therapeutics, as several drug targets the proteins involved in drug transport and metabolism exhibit daily rhythmic expression.63 Chronotherapy is an emerging therapeutic approach for COVID-19 and new drugs that are currently used in the treatment protocols for SARS-COV-2 have circadian properties.64 Therefore, another aspect of COVID-19 management will be to understand the dosing-time dependency of drugs that inhibit SARS-CoV-2 and elicit any clinical improvement in infected patients. For instance, it has been shown that corticosteroids reduce mortality in hospitalized COVID-19 patients.65
Nevertheless, authors found that administration of prednisolone is more beneficial in the evening compared to the morning in other diseases like rheumatoid arthritis and that might be applicable for COVID-19 treatment.65 However, there are no published clinical trials that have evaluated the circadian influence of prednisone or methylprednisolone against SARS-CoV-2, and therefore future studies should aim to explore this knowledge gap.
Further studies are also needed to examine the effect of circadian and sleep on the efficacy of vaccination in general and specifically for COVID-19 vaccination. Improving sleep quality and its circadian timing can potentially play a role in improving vaccination outcomes.
Sleep disturbances in children and adolescents during the COVID-19 pandemic
Children and adolescents require good quality sleep to ensure their well-being, adequate growth, and puberty development. Sleep disturbances are not infrequent in children. With the modern lifestyle of social media and increasing time spent on electronic devices, more and more children are suffering from sleep disturbances according to the American Academy of Sleep Medicine in 2014.66 The spectrum includes difficulties falling asleep, maintaining sleep, and/or waking too early. In children before the COVID-19 pandemic, this prevalence was as high as one in four children.67,68
The COVID-19 pandemic has affected all age groups although initially it was thought that children have mild disease with subsequent mutations and different strains, children have been reported to develop significant morbidities. It can cause serious severe symptoms and even death. SARS-CoV-2 can also cause neurological complications in children, such as shortness of breath, myalgia, stroke, and encephalopathy. These problems are highly linked with cytokine storm and proinflammatory responses, which can alter the physiology of the blood-brain-barrier and allow the virus to enter the brain.69
Sleep disturbances during COVID-19 illness and post-infection have been reported widely for adults and there is significant literature describing all aspects of this important topic. However, in children the studies are limited. The COVID-19 pandemic has influenced sleep patterns in all age groups. Although there are few reports of positive influence many have reported negative effects. Liu et al,70 in a study showed that almost 70% of the adult sample had poor sleep quality, with higher sleep latency and poor efficiency. It was noted for children aged 4–12 years the main issue was initiating and/or maintaining sleep, and for the 0–3 years group increased nocturnal awakenings. The authors have also found that sex and having a child with sleep disturbances significantly predicted parents’ sleep problems. The lockdown during the COVID-19 pandemic has been linked to both positive and negative changes in children’s sleep, specifically in terms of shifting sleep and wake times, increased nightmares, or increased sleep duration.70,71 While the delayed onset of sleep and later bedtime, which worsened during COVID-19, can be considered a sign of worsened sleep, in instances where children previously had insufficient hours of sleep, this may be considered a positive outcome.70 The positive or negative effects on sleep may be influenced by screen time, available social opportunities, physical activity levels, nutrition and diet, and increased flexibility in daily schedules.71–73 During the lockdown, the negative impact of these factors was intensified due to the limited time and opportunities for children to play and socialize.71 New research has demonstrated that poor sleep is also associated with reduced social interaction, by way of low mood.74
In addition to parent-specific challenges, sleep quality in parents and children demonstrates bidirectional associations, such that parents’ poor sleep and stress affect the child and vice versa.75–77 This can be exacerbated by disrupted circadian rhythms from inconsistent routines and lifestyle changes during the pandemic. A systemic review showed a longer duration of sleep time, an increase in sleep latency, and daytime sleepiness. However, it is still unknown if the adverse changes to sleep patterns and bedtime routines seen during the lockdown will have any long-term consequences for children’s sleep and daytime functioning.78
A UK study that involved 17 000 school-aged children and adolescents during COVID-19 restrictions, sleep patterns consistent with adolescent delayed sleep phase were observed, with longer sleep times for secondary school students. Perceived deteriorations in sleep quality were associated with reductions in happiness and interpersonal functioning, highlighting the importance of including sleep measures in adolescent well-being research.79
COVID-19 infection and the effect on the immune system of children
In general, COVID-19 infection in children causes mild and recoverable symptoms, however, it can cause serious severe symptoms and death. The cytokine storm and proinflammatory responses are linked to severe morbidities. In severe COVID-19 infection, there is a high risk of acute respiratory distress syndrome and multiorgan dysfunction and this has been attributed to the cytokine storm or substantial pro-inflammatory response.80 In some instances when cytokine expression is not elevated in peripheral blood immune cells, type I interferon expression plays an important role.80,81 Proinflammatory cytokines associated with SARS-CoV-2 include interferon gamma, TNF-α, vascular endothelial growth factor, monocyte chemotactic protein-1/C-C motif chemokine ligand (CCL)-2, ILs (IL-1Rα, IL-1β, IL-7, IL-8, and IL-10), granulocyte colony-stimulating factor (G-CSF), basic fibroblast growth factor, granulocyte-macrophage colony-stimulating factor, induced protein-10/CXCL10, macrophage inflammatory protein-1α/CCL3, macrophage inflammatory protein-1β/CCL4, and platelet-derived growth factor.82
Data regarding the immune response in children with COVID-19 infection is limited. However, the high CRP and IL-6 levels in children, suggest that the proinflammatory state in children may be like that in adults with COVID-19 infection.69
Effect of sleep disturbance on health care personnel
Studies investigated the influence of atypical work schedules on HCWs sleep in the context of the COVID-19 pandemic. It has been shown by several studies that there is an association between shift work and poor sleep quality among HCWs.83–86 One study found that in models adjusted for age, sex, profession, and country, individuals working nights shifts were 1.81 times more likely to sleep less than six hours per day than those who were not working night shifts.63 Furthermore, HCWs were subjected to longer working hours during the pandemic and consequently inadequate sleep.87 Many studies around the world have documented an increased prevalence of anxiety and feeling of hopelessness among frontline HCWs.88 Nurses in ICU and emergency department reported more psychological stress and lack of support from organizations and coping strategies. Similar complaints were reported from physicians in direct contact with COVID-19 patients.89 They also reported exhaustion and higher stress levels compared to their colleagues in non-COVID-19 wards. Other HCWs reported lack of personal protective equipment, heavy workload, and lack of community support.90
OSAS and COVID-19
OSAS is a common sleep-breathing disorder affecting around 2–4% of the world population.91 It is characterized by repetitive obstruction of the upper airway during sleep associated with desaturation. OSAS is also associated with excessive daytime sleepiness and impairment of cognitive functions.92 Several studies indicated that OSAS is associated with hypertension, ischemic heart diseases,93 and impairment of glycemic control, and these comorbidities are associated with unfavorable outcomes of COVID-19 disease. Studies have shown that about 70% of OSAS patients are obese and the opposite is also true (i.e., 40% of obese patients have OSAS). Studies have shown that OSAS is linked to pneumonia severity, it can increase aspiration and may compromise the efficacy of the defensive cough reflex. COVID-19 patients with OSA are at a higher risk of ICU admission, mechanical ventilation, and poor disease outcome.94
Several mechanisms are responsible for higher rates of disease complications in patients affected by OSAS [Figure 1]. We have indicated earlier that patients with severe OSAS have impaired immune response. OSAS may be associated with inflammatory and pro-Th2 immune responses, increased proliferative potential of NK, and CD4 T-cells.14 The changes in lymphocytic cells phenotypes associated with OSAS may also contribute to systemic inflammation.95 Furthermore, OSA can become a risk factor for ICU admission and it is associated with higher concentration of plasminogen activator inhibitor-1, a component of the coagulation system correlated with increased risk for acute vascular events.96 OSAS and obesity (hypoventilation) are associated with hypoxemia, which can be a worsening factor in the hypoxemia of COVID-19 pneumonia. It shows that OSAS may cause ventilatory compromise, which would impair ventilation in patients with COVID-19 disease.97 A supine position may cause lower airway obstruction and reduced forced vital capacity in obese patients with OSAS.98
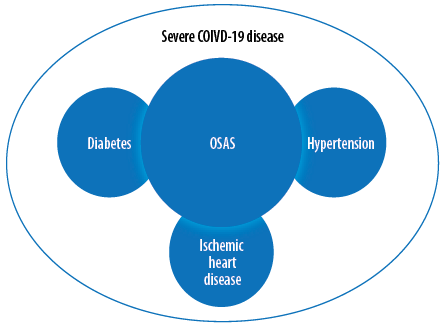 Figure 1: Association between obstructive sleep apnea syndrome (OSAS) and severe COVID-19 disease.
Figure 1: Association between obstructive sleep apnea syndrome (OSAS) and severe COVID-19 disease.
Conclusion
Sleep and immune response are connected through the circadian clock. Sleep disturbances and deprivation may depress the immune response to invading antigens like SARS-CoV-2. Proper sleep hygiene will keep the balance of humeral and cellular functions, which are required for better immune response to the disease and could help in recovery. There are multiple factors that modulate sleep and immune responses and that might influence the severity of COVID-19. IL-6 and melatonin are two of the main molecules among these factors. The relationship between sleep and immune response may also have a role in the severity of COVID-19 in children and adolescents and that could be similar in the adult population. OSA is a common sleep disorder and has a potential role in poor outcomes of COVID-19 disease and it interacts with other known risk factors such as hypertension and diabetes.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol 2004 Jun;4(6):457-467.
- 2. Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol 2005 Dec;116(6):1188-1198.
- 3. Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 1960;25:11-28.
- 4. van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res Brain Res Rev 2000 Aug;33(1):34-77.
- 5. Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci 1999 Feb;19(3):1072-1087.
- 6. Reid KJ, McGee-Koch LL, Zee PC. Cognition in circadian rhythm sleep disorders. Progress in Brain Research 2011;190:3-20.
- 7. Yuan Y, Wu S, Li W, He W. A Tissue-specific rhythmic recruitment pattern of leukocyte subsets. Front Immunol 2020 Feb;11:102.
- 8. Lumpkin MD. Neuroimmunology: an overview. Neuroscience in Medicine 2008:671-675.
- 9. Krueger JM, Takahashi S, Kapás L, Bredow S, Roky R, Fang J, et al. Cytokines in sleep regulation. Adv Neuroimmunol 1995;5(2):171-188.
- 10. Opp MR, Krueger JM. Sleep and immunity: a growing field with clinical impact. Brain Behav Immun 2015 Jul;47:1-3.
- 11. Said EA, Al-Abri MA, Al-Saidi I, Al-Balushi MS, Al-Busaidi JZ, Al-Reesi I, et al. Altered blood cytokines, CD4 T cells, NK and neutrophils in patients with obstructive sleep apnea. Immunol Lett 2017 Oct;190:272-278.
- 12. Uchino E, Sonoda S, Kinukawa N, Sakamoto T. Alteration pattern of tear cytokines during the course of a day: diurnal rhythm analyzed by multicytokine assay. Cytokine 2006 Jan;33(1):36-40.
- 13. Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun 2005 Mar;19(2):165-172.
- 14. Born J, Lange T, Hansen K, Mölle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 1997 May;158(9):4454-4464.
- 15. Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun 2002 Oct;16(5):503-512.
- 16. Axelsson J, Rehman JU, Akerstedt T, Ekman R, Miller GE, Höglund CO, et al. Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/ T helper 2 balance in humans. PLoS One 2013 Dec;8(12):e82291.
- 17. Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab 2000 Oct;85(10):3597-3603.
- 18. Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 1999 Aug;84(8):2603-2607.
- 19. Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Annals of Internal Medicine 1998;128(2):127-137.
- 20. Nosetti LM, Milan V, Gaiazzi M, Di Maio RC, Marino F, Cosentino M, et al. Circadian rhythms of inflammatory markers in pediatric obstructive sleep apnea syndrome. InC96. American Journal of Respiratory and Critical Care Medicine 2011;183:A5325.
- 21. Li Y, Vgontzas AN, Fernandez-Mendoza J, Kritikou I, Basta M, Pejovic S, et al. Objective, but not subjective, sleepiness is associated with inflammation in sleep apnea. Sleep 2017 Feb;40(2):zsw033.
- 22. Said EA, Al-Abri MA, Al-Saidi I, Al-Balushi MS, Al-Busaidi JZ, Al-Reesi I, et al. Sleep deprivation alters neutrophil functions and levels of Th1-related chemokines and CD4+ T cells in the blood. Sleep Breath 2019 Dec;23(4):1331-1339.
- 23. Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev 1996 Feb;17(1):64-102.
- 24. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun 2007 Nov;21(8):1050-1057.
- 25. Liu T, Zhang J, Yang Y, Zhang L, Ma H, Li Z, et al. The potential role of IL-6 in monitoring coronavirus disease 2019. MedRxiv 2020.
- 26. Zhang J, Xu D, Xie B, Zhang Y, Huang H, Liu H, et al. Poor-sleep is associated with slow recovery from lymphopenia and an increased need for ICU care in hospitalized patients with COVID-19: a retrospective cohort study. Brain Behav Immun 2020 Aug;88:50-58.
- 27. Chhetri S, Khamis F, Pandak N, Al Khalili H, Said E, Petersen E. A fatal case of COVID-19 due to metabolic acidosis following dysregulate inflammatory response (cytokine storm). IDCases 2020 May;21:e00829.
- 28. Said EA, Al-Reesi I, Al-Shizawi N, Jaju S, Al-Balushi MS, Koh CY, et al. Defining IL-6 levels in healthy individuals: a meta-analysis. J Med Virol 2021 Jun;93(6):3915-3924.
- 29. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020 Jun;395(10239):1763-1770.
- 30. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020 Aug;324(8):782-793.
- 31. Zhang J, Hao Y, Ou W, Ming F, Liang G, Qian Y, et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med 2020 Oct;18(1):406.
- 32. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically Ill patients with covid-19. N Engl J Med 2021 Apr;384(16):1491-1502.
- 33. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med 2021 Jan;384(1):20-30.
- 34. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al; RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med 2020 Nov;383(21):2030-2040.
- 35. Veiga VC, Prats JA, Farias DL, Rosa RG, Dourado LK, Zampieri FG, et al; Coalition covid-19 Brazil VI Investigators. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021 Jan;372(84):n84.
- 36. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008 Jan;9(1):46-56.
- 37. Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci 2012 Jul;1261:88-96.
- 38. Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, et al. Sleep enhances IL-6 trans-signaling in humans. FASEB J 2006 Oct;20(12):2174-2176.
- 39. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015 May;16(5):448-457.
- 40. Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation 2011 May;8:54.
- 41. Fried EI, von Stockert S, Haslbeck JM, Lamers F, Schoevers RA, Penninx BW. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol Med 2020 Dec;50(16):2682-2690.
- 42. Jokela M, Virtanen M, Batty GD, Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiatry 2016 Jan;73(1):87-88.
- 43. Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry 2021 Dec;26(12):7393-7402.
- 44. Moriarity DP, Horn SR, Kautz MM, Haslbeck JM, Alloy LB. How handling extreme C-reactive protein (CRP) values and regularization influences CRP and depression criteria associations in network analyses. Brain Behav Immun 2021 Jan;91:393-403.
- 45. White J, Kivimäki M, Jokela M, Batty GD. Association of inflammation with specific symptoms of depression in a general population of older people: the English longitudinal study of ageing. Brain Behav Immun 2017 Mar;61:27-30.
- 46. Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021 Mar;11(3):e048391.
- 47. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021 May;8(5):416-427.
- 48. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 2021 Apr;27(4):601-615.
- 49. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al; COVID-19 BioB Outpatient Clinic Study group. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020 Oct;89:594-600.
- 50. Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun 2021 May;94:138-147.
- 51. De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 2020 Jul;11(1):3434.
- 52. Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021 Jan;137:155323.
- 53. Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-Reactive CD4+ T cells in COVID-19. Cell 2020 Nov;183(5):1340-1353.e16.
- 54. Lin X, Liu Y, Ma L, Ma X, Chen Z, Chen H, et al. Amelioration of experimental autoimmune encephalomyelitis by Rhodiola rosea, a natural adaptogen. Biomed Pharmacother 2020 May;125:109960.
- 55. Bhattacharya S, Patel KK, Dehari D, Agrawal AK, Singh S. Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem 2019 Dec;462(1-2):133-155.
- 56. Ferlazzo N, Andolina G, Cannata A, Costanzo MG, Rizzo V, Currò M, et al. Is melatonin the cornucopia of the 21st century? Antioxidants (Basel) 2020 Nov;9(11):E1088.
- 57. Boga JA, Coto-Montes A, Rosales-Corral SA, Tan D-X, Reiter RJ. Beneficial actions of melatonin in the management of viral infections: a new use for this “molecular handyman”? Rev Med Virol 2012 Sep;22(5):323-338.
- 58. Zhou Y, Hou Y, Shen J, Mehra R, Kallianpur A, Culver DA, et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol 2020 Nov;18(11):e3000970-e3000970.
- 59. Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv 2020 Jan 1;2020.
- 60. Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with covid-19: a randomized, double-blind clinical trial. Arch Med Res 2022 Jan;53(1):79-85.
- 61. Alizadeh Z, Keyhanian N, Ghaderkhani S, Dashti-Khavidaki S, Shoormasti RS, Pourpak Z. A pilot study on controlling coronavirus disease 2019 (covid-19) inflammation using melatonin supplement. Iran J Allergy Asthma Immunol 2021 Aug 7;20(4 SE-Brief Communication):1-6.
- 62. Mousavi SA, Heydari K, Mehravaran H, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, et al. Melatonin effects on sleep quality and outcomes of COVID-19 patients: an open-label, randomized, controlled trial. J Med Virol 2022 Jan;94(1):263-271.
- 63. Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science 2019 Aug;365(6453):547-549.
- 64. Giri A, Srinivasan A, Sundar IK. COVID-19: sleep, circadian rhythms and immunity - repurposing drugs and chronotherapeutics for SARS-CoV-2. Front Neurosci 2021 Jun;15:674204.
- 65. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19. N Engl J Med 2021 Feb;384(8):693-704.
- 66. Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007 Nov;30(11):1445-1459.
- 67. Mindell JA, Kuhn B, Lewin DS, Meltzer LJ, Sadeh A; American Academy of Sleep Medicine. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 2006 Oct;29(10):1263-1276.
- 68. Williamson AA, Mindell JA, Hiscock H, Quach J. Child sleep behaviors and sleep problems from infancy to school-age. Sleep Med 2019 Nov;63:5-8.
- 69. Khan S, Siddique R, Hao X, Lin Y, Liu Y, Wang X, et al. The COVID-19 infection in children and its association with the immune system, prenatal stress, and neurological complications. Int J Biol Sci 2022 Jan;18(2):707-716.
- 70. Liu Z, Tang H, Jin Q, Wang G, Yang Z, Chen H, et al. Sleep of preschoolers during the coronavirus disease 2019 (COVID-19) outbreak. J Sleep Res 2021 Feb;30(1):e13142.
- 71. Moore SA, Faulkner G, Rhodes RE, Brussoni M, Chulak-Bozzer T, Ferguson LJ, et al. Impact of the COVID-19 virus outbreak on movement and play behaviours of Canadian children and youth: a national survey. Int J Behav Nutr Phys Act 2020 Jul;17(1):85.
- 72. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020 Mar;395(10227):912-920.
- 73. Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (covid-19) epidemic among the general population in China. Int J Environ Res Public Health 2020 Mar;17(5):E1729.
- 74. Wakefield JR, Bowe M, Kellezi B, Butcher A, Groeger JA. Longitudinal associations between family identification, loneliness, depression, and sleep quality. Br J Health Psychol 2020 Feb;25(1):1-16.
- 75. Orgilés M, Morales A, Delvecchio E, Mazzeschi C, Espada JP. Immediate psychological effects of the COVID-19 quarantine in youth from Italy and Spain. Front Psychol 2020 Nov;11:579038.
- 76. Prime H, Wade M, Browne DT. Risk and resilience in family well-being during the COVID-19 pandemic. Am Psychol 2020 Jul-Aug;75(5):631-643.
- 77. Varma P, Conduit R, Junge M, Jackson ML. Examining sleep and mood in parents of children with sleep disturbances. Nat Sci Sleep 2020 Oct;12:865-874.
- 78. Camacho-Montaño LR, Iranzo A, Martínez-Piédrola RM, Camacho-Montaño LM, Huertas-Hoyas E, Serrada-Tejeda S, et al. Effects of COVID-19 home confinement on sleep in children: a systematic review. Sleep Med Rev 2022 Apr;62:101596.
- 79. Illingworth G, Mansfield KL, Espie CA, Fazel M, Waite F. Sleep in the time of COVID-19: findings from 17000 school-aged children and adolescents in the UK during the first national lockdown. Sleep Adv a J Sleep Res Soc. 2022;3(1):zpab021.
- 80. Molloy EJ, Bearer CF. COVID-19 in children and altered inflammatory responses. Vol. 88. Pediatric research: United States; 2020. p. 340-341.
- 81. Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest 2020 Nov;130(11):5967-5975.
- 82. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Feb;395(10223):497-506.
- 83. Arafa A, Mohammed Z, Mahmoud O, Elshazley M, Ewis A. Depressed, anxious, and stressed: what have healthcare workers on the frontlines in Egypt and Saudi Arabia experienced during the COVID-19 pandemic? J Affect Disord 2021 Jan;278:365-371.
- 84. Luo M, Guo L, Yu M, Jiang W, Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and general public - a systematic review and meta-analysis. Psychiatry Res 2020 Sep;291:113190.
- 85. Kang L, Ma S, Chen M, Yang J, Wang Y, Li R, et al. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross-sectional study. Brain Behav Immun 2020 Jul;87:11-17.
- 86. Tan BY, Chew NW, Lee GK, Jing M, Goh Y, Yeo LL, et al. Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann Intern Med 2020 Aug;173(4):317-320.
- 87. Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open 2020 Mar;3(3):e203976.
- 88. Liu C-Y, Yang Y-Z, Zhang X-M, Xu X, Dou Q-L, Zhang W-W, et al. The prevalence and influencing factors in anxiety in medical workers fighting COVID-19 in China: a cross-sectional survey. Epidemiol Infect 2020 May;148:e98.
- 89. Zerbini G, Ebigbo A, Reicherts P, Kunz M, Messman H. Psychosocial burden of healthcare professionals in times of COVID-19 - a survey conducted at the University Hospital Augsburg. Ger Med Sci 2020 Jun;18:Doc05.
- 90. Razu SR, Yasmin T, Arif TB, Islam MS, Islam SMS, Gesesew HA, et al. Challenges faced by healthcare professionals during the covid-19 pandemic: a qualitative inquiry from Bangladesh. Front public Heal 2021;1024.
- 91. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002 May;165(9):1217-1239.
- 92. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med 2017 Mar;13(3):479-504.
- 93. Cross MD, Mills NL, Al-Abri M, Riha R, Vennelle M, Mackay TW, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trial. Thorax 2008 Jul;63(7):578-583.
- 94. Miller MA, Cappuccio FP. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev 2021 Feb;55:101382.
- 95. Tan H-L, Gozal D, Wang Y, Bandla HP, Bhattacharjee R, Kulkarni R, et al. Alterations in circulating T-cell lymphocyte populations in children with obstructive sleep apnea. Sleep 2013 Jun;36(6):913-922.
- 96. Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013 Mar;37(3):333-340.
- 97. Rögnvaldsson KG, Eyþórsson ES, Emilsson ÖI, Eysteinsdóttir B, Pálsson R, Gottfreðsson M, et al. Obstructive sleep apnea is an independent risk factor for severe COVID-19: a population-based study. Sleep 2022 Mar;45(3):zsab272.
- 98. Al Lawati R, Al Abri MA, Kuppuswamy B, Al-Kharousi A, Al-Atbi AY, Rizvi S, et al. The effect of change in posture on spirometry in patients with obstructive sleep apnoea syndrome. Sultan Qaboos Univ Med J 2019 Nov;19(4):e310-e315.