The definition of acute respiratory distress syndrome (ARDS) has evolved since it was first described in 1967. The 2011 Berlin definition takes into consideration the timing of the acute onset, chest imaging, origin of edema, and eliminating the term acute lung injury.1 ARDS is one of the commonest conditions encountered in intensive care worldwide and accounts for nearly 10–15% of intensive care unit (ICU) admissions.2–5 It has been associated with a mortality rate of 40% and numerous long-term complications.6–8 Approximately 40% of patients with COVID-19 pneumonia develop ARDS within 8–10 days following symptom onset.9–13 Mortality rate of 26% was reported in patients with COVID-19 pneumonia that got admitted to the ICU and required mechanical ventilation ranges.10,11
Due to diffuse alveolar damage in COVID-19 patients, hypoxemic respiratory failure develops; however, atypical changes, such as thrombosis and disseminated intravascular coagulation have also been reported.14–17
Prone positioning is one of the measures that has been used over the last five decades to improve the outcomes of patients with severe ARDS and refractory hypoxemia.18–22 In 2013, the PROSEVA study showed that prone positioning improved the survival rate of moderate to severe ARDS patients with a ratio of PaO2 to FiO2 of < 150.23 In addition, several meta-analyses showed favorable outcomes in patients with ARDS who are promptly placed in the prone position for a prolonged period.24–30 The beneficial effects of prone positioning were attributed to improvements in gas exchange, respiratory system compliance, and lung protection.31 Despite these benefits, studies have demonstrated that prone positioning has not been integrated into routine therapy for most ARDS patients worldwide.32,33
Early studies and international societies supported the use of the prone position in COVID-19-related ARDS.34–37 However, there is insufficient evidence supporting the beneficial effect of prone positioning on moderate to severe ARDS due to COVID-19 infection. Although, most of the time, COVID pneumonia falls under the Berlin definition of ARDS, severe hypoxemia associated with near-normal respiratory system compliance was distinctively observed in COVID pneumonia, which resulted in striking non-uniformity in the course of the disease and response to management.38 With this in mind, different COVID-19 patterns may be found at presentation that is explained by the development of a time-related disease spectrum within two primary phenotypes. The ‘L-type’ ARDS is characterized by low lung elasticity, weight, and lung recruitability. Meanwhile, the ‘H-type’ is characterized by high lung elasticity, intra-pulmonary right-to-left shunting, lung weight, and lung recruitability.37–39 Earlier in the course of the disease, ‘L-type’ takes place and may remain unchanging for a period and then improve or worsen to ‘H-type’. Therefore, not all COVID-19 patients benefit from higher levels of positive end-expiratory pressure (PEEP) and prone positioning. We conducted a retrospective study with the aim of determining the effect of early prone positioning on oxygenation and ultimately mortality of moderate to severe COVID-19 ARDS patients.
Methods
This was a retrospective study involving COVID-19-related ARDS patients who were intubated and underwent mechanical ventilation with a PaO2/FiO2 ratio of < 150, in the respective ICUs of Khoula Hospital and Sultan Qaboos University Hospital (SQUH) between 1 May and 31 October 2020. Ethical approval was obtained from the Medical Research Ethics Committee at the SQUH (SQU-EC/328/2021) and the Research and Ethical Review and Approval Committee of the Ministry of Health Oman (MoH/CSR/20/24201). Written informed consent was not required and was waived as it was a retrospective study. Ethical approval was obtained from the ethical committees at both hospitals.
The study included patients who were 18 years old or older; had infection with COVID-19 confirmed via polymerase chain reaction within the last 15 days, were intubated and underwent mechanical ventilation in the previous 48 hours, had a PaO2/FiO2 ratio < 150 on FiO2 60% or more, and PEEP of > 8 cm H2O.
Patients were excluded if they underwent invasive mechanical ventilation for > 48 hours prior to prone positioning, had a PaO2/FiO2 ratio of > 150, or were re-intubated.
Categorical variables were summarized using frequencies and percentages, while differences between groups were analyzed using Pearson’s χ2 test (or Fisher’s exact test for expected cells < 5). Continuous variables such as age and body mass index (BMI) were presented as means and SDs, and analyses were performed using Student’s t-test. Continuous but abnormally distributed variables (assessed using the Kolmogorov-Smirnov test) such as APACHE II scores, sequential organ failure assessment (SOFA) scores, and symptoms days were presented as medians and interquartile ranges and analyzed using the Wilcoxon-Mann-Whitney test. Ventilator parameters (pH, pO2, and P/F ratio [arterial pO2 divided by the FiO2]) of the patients throughout their ICU admission (days 1–4 of intubation) between supine and prone sleeping positions, as presented in Figures 1–3, were analyzed using the repeated measures analysis of variance. P-values (two-sided) for the differences over time were corrected using the Greenhouse-Geisser correction factor. Statistical analyses were conducted using StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.
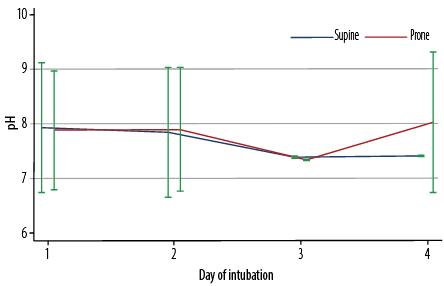 Figure 1: Arterial pH values from day one to day four of intubation, stratified by position (supine or prone) (N = 235).
Figure 1: Arterial pH values from day one to day four of intubation, stratified by position (supine or prone) (N = 235).
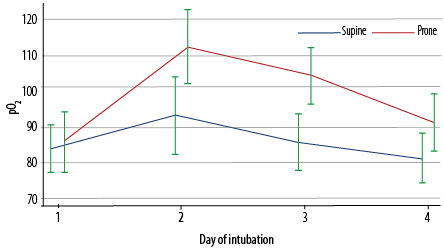 Figure 2: Arterial pO2 values from day one to day four of intubation, stratified by position (supine or prone) (N = 235).
Figure 2: Arterial pO2 values from day one to day four of intubation, stratified by position (supine or prone) (N = 235).
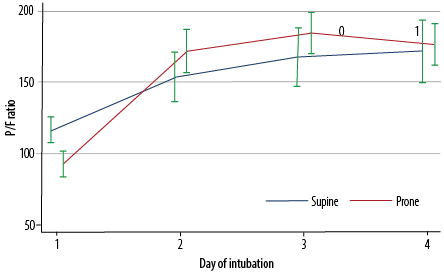 Figure 3: P/F ratio from day one to day four of intubation, stratified by position (supine or prone) (N = 235).
Figure 3: P/F ratio from day one to day four of intubation, stratified by position (supine or prone) (N = 235).
Results
A total of 310 patients were admitted to the ICUs during the six-month study. Forty-five patients were transferred to other hospitals and 17 died within 24 hours of ICU admission. A total of 248 patients were analyzed. Among them, 166 were admitted to the ICU at SQUH, and 82 were admitted to the ICU at Khoula hospital.
The demographic and clinical characteristics of the patients are listed in Table 1. The mean age was 56.0±15.0 years, 70.6% (n = 166) were male, and 13.0% (n = 30/230) were current smokers. The overall mean BMI was 31.5±7.5 kg/m2. The most prevalent comorbidities were diabetes mellitus (55.7%; n = 131), hypertension (45.5%; n = 107), and coronary artery disease (10.6%; n = 25). The most frequent presenting symptoms were fever (81.3%; n = 191), shortness of breath (73.6%; n = 173), and cough (63.0%; n = 148). The median length of symptoms was 5 (3–8) days.
Table 1: Demographic and clinical characteristics of patients stratified by groups (supine or prone).
|
Demographic
|
|
|
|
|
|
Age, mean ± SD, years (n = 234)
|
56.0 ± 15.0
|
57.0 ± 16.0
|
55.0 ± 15.0
|
0.432
|
|
Male sex
|
166 (70.6)
|
78 (67.8)
|
88 (73.3)
|
0.354
|
|
BMI, mean ± SD, kg/m2 (n = 32)
|
31.5 ± 7.5
|
34.6 ± 10.0
|
30.3 ± 6.2
|
0.145
|
|
Current smoker (n = 230)
|
30 (13.0)
|
11 (9.6)
|
19 (15.8)
|
0.130
|
|
Clinical
|
|
|
|
|
|
Hypertension
|
107 (45.5)
|
57 (49.6)
|
50 (41.7)
|
0.224
|
|
Diabetes mellitus
|
131 (55.7)
|
68 (59.1)
|
63 (52.5)
|
0.306
|
|
Coronary artery disease
|
25 (10.6)
|
8 (7.0)
|
17 (14.2)
|
0.073
|
|
Chronic kidney disease
|
19 (8.1)
|
7 (6.1)
|
12 (10.0)
|
0.271
|
|
Chronic lung disease
|
11 (4.7)
|
7 (6.1)
|
4 (3.3)
|
0.368
|
|
Malignancy
|
7 (3.0)
|
2 (1.7)
|
5 (4.2)
|
0.447
|
|
Symptoms at presentation
|
|
|
|
|
|
Fever
|
191 (81.3)
|
87 (75.7)
|
104 (86.7)
|
0.030
|
|
Cough
|
148 (63.0)
|
71 (61.7)
|
77 (64.2)
|
0.700
|
|
Shortness of breath
|
173 (73.6)
|
78 (67.8)
|
95 (79.2)
|
0.049
|
|
Chest pain
|
17 (7.2)
|
10 (8.7)
|
7 (5.8)
|
0.397
|
|
Abdominal symptoms
|
40 (17.0)
|
33 (28.7)
|
7 (5.8)
|
< 0.001
|
|
Neurological symptoms
|
28 (11.9)
|
16 (13.9)
|
12 (10.0)
|
0.355
|
|
Trauma
|
2 (0.9)
|
1 (0.9)
|
1 (0.8)
|
0.976
|
|
Severity scores on admission, median (IQR)
|
|
|
|
|
|
APACHE II scores
|
20 (13–22)
|
13 (9–20)
|
20 (18–22)
|
< 0.001
|
|
SOFA scores (n = 234)
|
5 (3–5)
|
4 (3–5)
|
5 (4.5–5)
|
< 0.001
|
|
Symptoms, days (n = 227)
|
5 (3–8)
|
5 (3–7)
|
5 (3–8)
|
0.495
|
|
Chest radiography findings (n = 231)
|
|
|
|
|
|
Focal consolidation
|
13 (5.5)
|
11 (9.6)
|
2 (1.7)
|
0.007
|
|
Bilateral lung infiltrates
|
216 (91.9)
|
99 (86.1)
|
117 (987.5)
|
0.002
|
|
Pulmonary edema
|
2 (0.9)
|
2 (1.7)
|
0 (0.0)
|
0.234
|
|
Vasopressors (n = 232)
|
164 (70.7)
|
81 (70.4)
|
83 (69.2)
|
0.905
|
|
Renal replacement therapy
|
44 (19.0)
|
14 (12.2)
|
30 (25.0)
|
0.010
|
|
NIV before intubation
|
107 (45.5)
|
26 (22.6)
|
81 (67.5)
|
< 0.001
|
|
Laboratory investigations, mean ± SD, years
|
|
|
|
|
|
CRP, mg/L (n = 234)
|
156.0 ± 108.0
|
167.0 ± 111.0
|
146.0 ± 105.0
|
0.137
|
|
D-dimer, ng/mL (n = 231)
|
7.0 ± 15.0
|
7.4 ± 15.0
|
6.7 ± 15.0
|
0.713
|
|
Ferritin, ng/mL (n = 232)
|
2643.0 ± 6806.0
|
2390.0 ± 6033.0
|
2889.0 ± 7496.0
|
0.578
|
|
LDH (n = 228)
|
620.0 ± 401.0
|
647.0 ± 277.0
|
594.0 ± 489.0
|
0.323
|
|
IL-6, pg/mL (n = 150)
|
283.0 ± 651.0
|
446.0 ± 903.0
|
141.0 ± 207.0
|
0.004
|
|
Management
|
|
|
|
|
|
Steroids (n = 233)
|
225 (96.6)
|
111 (96.5)
|
114 (95.0)
|
0.970
|
|
Antivirals (remdesivir) (n = 228)
|
10 (4.4)
|
6 (5.2)
|
4 (3.3)
|
0.748
|
|
IL (anakinra/tocilizumab) (n = 232)
|
43 (18.5)
|
19 (16.5)
|
24 (20.0)
|
0.472
|
BMI: body mass index; SOFS: sequential organ failure assessment; IQR: interquartile range; NIV: non-invasive ventilation; CRP: C-reactive protein; LDH, lactate dehydrogenase; IL: interleukin.
Data were given as n (%) unless specified otherwise.
On admission, the median APACHE II and SOFA scores were 20 (13–22) and 5 (3–5), respectively. Higher APACHE II (20 vs. 13; p < 0.001) and SOFA (5 vs. 4; p < 0.001) scores were observed in patients underwent prone positioning than those who were not in the prone position. Chest radiography findings revealed bilateral lung infiltrates in most patients (91.9%; n = 216) and pulmonary edema in two patients (0.9%). A total of 70.7% (n = 164/232) and 19.0% (44/231) of the patients had vasopressor and renal replacement therapy (RRT), respectively. A quarter (25.0%) of patients who were prone-positioned also required RRT while 12.0% (p = 0.010) in the supine position required RRT.
Of the patients prone-positioned, 67.5% received non-invasive ventilation before intubation compared with 22.6% in the supine group. In terms of management, 96.6% (225/233) received steroids, 18.5% (43/232) received anakinra/tocilizumab, 7.4% (17/229) received convalescent plasma, and 4.4% (10/228) received remdesivir. The decision on giving this medical treatment is based on the latest updated protocol at that time, while the ventilator settings are decided by the attending physician at the time of intubation. The parameters get adjusted after repeating arterial blood gas with lung protective measures mentioned in the ARDS protocol in mind. Adjustments could be made by the primary physician, respiratory therapist, or the consultant in charge.
As shown in Table 2, no significant differences in mortality (48.3% vs. 47.8%; p = 0.938) and discharge rates (50.8% vs. 51.3%; p = 0.942) were observed between the prone and supine groups. However, patients in the prone position were associated with a longer hospital stay (19 vs. 16 days; p = 0.041) and more frequent tracheostomies (30.0% vs. 9.6%;
p < 0.001) than those in the supine group, and these values of better statistical significance that may indirectly show a better outcome for patients kept in the prone position. To clarify, patients usually get tracheostomized after passing the critical stage of the disease and going into the weaning phase of mechanical ventilation, which requires longer hospital stay.
Table 2: Outcome characteristics stratified by groups (supine or prone).
|
Primary
|
|
Mortality
|
113 (48.1)
|
55 (47.8)
|
58 (48.3)
|
0.938
|
|
Discharged
|
120 (51.1)
|
59 (51.3)
|
61 (50.8)
|
0.942
|
|
Discharged to inpatient
|
2 (0.9)
|
1 (0.9)
|
1 (0.8)
|
1.000
|
|
Secondary
|
|
LOS, median (IQR), days
|
17 (11–30)
|
16 (10–26)
|
19 (12–34)
|
0.041
|
|
ICU LOS, median (IQR), days
|
10 (6–20)
|
11 (7–20)
|
9 (4–20)
|
0.257
|
ICU: intensive care unit; LOS: length of hospital; IQR: interquartile range.
ICU and hospital LOS were missing on one (234/235) and two (233/235) occasions, respectively.
Data were given as n (%) unless specified otherwise.
As illustrated in Figure 1, there were no significant differences in the arterial pH values between the prone and supine positions over time (days 1–4 of intubation). Figure 2 indicates the significant interaction in pO2 values between the sleeping position and duration of intubation (p = 0.041). While the values were similar on day one, the pO2 values thereafter (days 2–4) were significantly higher in patients in the prone position than in those in the supine position (p < 0.001). Furthermore, as shown in Figure 3, there was also a significant interaction between the position and the duration of intubation (p < 0.001). Patients in the prone position had a significantly lower PaO2/FiO2 ratio on day 1. However, their PaO2/FiO2 values surpassed those of patients in the supine position on days 2 and 3 (p < 0.001). The PaO2/FiO2 values of both groups were reduced to similar values on day 4.
With these p-values in mind, it is safe to say that prone position led to better oxygenation in early stages of the prone position with great statistical significance. Data were collected for four days, but the duration of prone position might extend to 8–12 days depending on many factors, including responsiveness to the prone position and hemodynamics. On the other hand, the data collected do not necessary correspond to four consecutive days.
Discussion
In this retrospective analysis, there was a significant improvement in oxygenation of the prone group, compared to the supine group. This finding supported the hypothesis that prone positioning improved ventilation-perfusion mismatch in ARDS patients.40,41 However, this did not improve the mortality rate. The mortality was 48.0% in both groups with poor statistical significance and the results are contrasting with what has been reported in non-COVID patients.
Previous studies in non-COVID-19 patients have reported the benefits of early prone positioning in ICU patients with a pO2/FiO2 ratio < 150, despite lung-protective ventilation and adequate PEEP.23 A recent meta-analysis showed that the PaO2/FiO2 ratio and oxygen saturation of COVID-19 patients improved with prone positioning, which resemble our findings.42 Another meta-analysis demonstrated that awake and non-intubated patients that underwent prone positioning had improved respiratory and intubation rates, compared to patients in the supine position.43
There were some limitations to our study. First, the sample size was small. Second, this was a retrospective study; therefore, selection bias may be present. Third, prone positioning was performed mainly in one hospital, while supine positioning was conducted primarily in the other hospital. Although the disease severity, degree of ARDS, and the number of involved organs were specified, other significantly different variables such as nurse-patient ratio, availability of consultant services and experience of prone positioning between the two hospitals may have been present. Fourth, lung-protective ventilation was applied according to the ideal body weight, and BMI was not recorded in all patients. Therefore, the results of this study may not be generalizable to other hospitals and other countries.
Further studies are required to compare the effects of early prone and supine positioning on moderate to severe ARDS in intubated COVID-19 pneumonia patients.
Conclusion
The findings of this retrospective study show that early prone position in COVID-19-related ARDS does not result in significant mortality benefit compared to the supine position. More research and randomized clinical trials are required in this regard.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis 2013 Jun;5(3):326-334.
- 2. Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 1983 May;98(5 Pt 1):593-597.
- 3. Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995 Feb;151(2 Pt 1):293-301.
- 4. Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, et al; ALIEN Network. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011 Dec;37(12):1932-1941.
- 5. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016 Feb;315(8):788-800.
- 6. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005 Oct;353(16):1685-1693.
- 7. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000 May;342(18):1301-1308.
- 8. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 2012 Mar;185(5):517-524.
- 9. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012 Jun;307(23):2526-2533.
- 10. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020 Jul;180(7):934-943.
- 11. Murthy S, Gomersall CD, Fowler RA. Care for critically Ill patients with COVID-19. JAMA 2020 Apr;323(15):1499-1500.
- 12. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020 May;8(5):475-481.
- 13. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020 Apr;8(4):420-422.
- 14. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 2020 Jun;33(6):1007-1014.
- 15. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 2020 Aug;30(8):4381-4389.
- 16. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 2020 Jul;18(7):1752-1755.
- 17. Mellins RB. Pulmonary physiotherapy in the pediatric age group. Am Rev Respir Dis 1974 Dec;110(6 Pt 2):137-142.
- 18. Piehl MA, Brown RS. Use of extreme position changes in acute respiratory failure. Crit Care Med 1976 Jan-Feb;4(1):13-14.
- 19. Gattinoni L, Carlesso E, Caironi P. Stress and strain within the lung. Curr Opin Crit Care 2012 Feb;18(1):42-47.
- 20. Tonetti T, Vasques F, Rapetti F, Maiolo G, Collino F, Romitti F, et al. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017 Jul;5(14):286.
- 21. Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997 Mar;99(5):944-952.
- 22. Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis 1977 Apr;115(4):559-566.
- 23. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013 Jun;368(23):2159-2168.
- 24. Abroug F, Ouanes-Besbes L, Elatrous S, Brochard L. The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: a meta-analysis. Areas of uncertainty and recommendations for research. Intensive Care Med 2008 Jun;34(6):1002-1011.
- 25. Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med 2008 Feb;36(2):603-609.
- 26. Lee JM, Bae W, Lee YJ, Cho YJ. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med 2014 May;42(5):1252-1262.
- 27. Hu SL, He HL, Pan C, Liu AR, Liu SQ, Liu L, et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care 2014 May;18(3):R109.
- 28. Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014 Mar;40(3):332-341.
- 29. Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol 2010 Jun;76(6):448-454.
- 30. Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010 Apr;36(4):585-599.
- 31. Chanques G, Constantin JM, Devlin JW, Ely EW, Fraser GL, Gélinas C, et al. Analgesia and sedation in patients with ARDS. Intensive Care Med 2020 Dec;46(12):2342-2356.
- 32. Guérin C, Beuret P, Constantin JM, Bellani G, Garcia-Olivares P, Roca O, et al; investigators of the APRONET Study Group, the REVA Network, the Réseau recherche de la Société Française d’Anesthésie-Réanimation (SFAR-recherche) and the ESICM Trials Group. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med 2018 Jan;44(1):22-37.
- 33. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019;380(21):1997-2008.
- 34. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med 2020 May;46(5):854-887.
- 35. Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020 May;8(5):506-517.
- 36. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med 2020 May;382(21):2012-2022.
- 37. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020 Apr;323(16):1545-1546.
- 38. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020 Jun;46(6):1099-1102.
- 39. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “ typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020 May;201(10):1299-1300.
- 40. Tan W, Xu DY, Xu MJ, Wang ZF, Dai B, Li LL, et al. The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: a meta-analysis. Ther Adv Respir Dis 2021 Jan-Dec;15:17534666211009407.
- 41. Kallet RH. A comprehensive review of prone position in ARDS. Respir Care 2015 Nov;60(11):1660-1687.
- 42. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020 Jun;323(22):2329-2330.
- 43. Chua EX, Zahir SM, Ng KT, Teoh WY, Hasan MS, Ruslan SR, et al. Effect of prone versus supine position in COVID-19 patients: a systematic review and meta-analysis. J Clin Anesth 2021 Nov;74:110406.