Endometriosis affects about 5–15% of women in reproductive age, impacting their quality of life (QoL).1–4 Presenting symptoms are wide-ranging and include infertility, dysmenorrhea, dyspareunia, chronic pelvic pain, and dyschezia.5 Endometriosis symptoms may overlap with other common gynecologic pathologies like adenomyosis and uterine leiomyoma.5–8 Early suspicion and diagnosis would minimize suffering and help improve QoL. In practice, however, many women suffer for years before being diagnosed and referred for endometriosis care.4,7–10 The mean delay in the diagnosis of endometriosis is reported to be 11.7 years in the US, 7.9 years in the UK,10.4 years in Germany, and 7.0 years in Brazil.11–13 Delay in diagnosis by laparoscopy for women with endometriosis from an Arab ancestry was found to be 11.61 years, and the average age at diagnosis was 34.04±4.81 years.11 Such long delays greatly prolong the suffering of the affected women, decrease their QoL and productivity, increase the risk of comorbidities, and add to the burden of the healthcare system.9,10
Most studies have estimated the delay in diagnosis of endometriosis using questionnaires to patients4,9,12–14 either self-administered, or interview-based (in-person, telephonic, or online). This method of information gathering has several issues depending on the method to recruit women, the mode of distribution of questionnaires, and the method of eliciting the answers. Cross-sectional surveys of women registered through endometriosis societies, interest groups, or self-help groups carry the risk of bias as such responders are more likely to have had severe symptoms or bad experiences with the medical profession or during the diagnosis process.15,16 In addition, targeting such demographies excludes women with mild or no symptoms.17 If the recruitment is hospital-based, even stronger bias in responses is likely, such as perception of more severe symptoms and overestimation of prevalence.18 When the recruitment is population-based, non-response bias becomes more evident. Regarding questions on the date of onset of symptoms, many patients experience recall bias.12,19,20 Furthermore, any lower abdominal pain or pelvic pain might be interpreted as a symptom of endometriosis.17,21 Thus, the questionnaire-based assessment of delayed diagnosis of endometriosis carries a significant risk of biased and missed data.17
Despite the possible biases, personal interviews are likely to provide more accurate information than self-answered questionnaires.22 We can regard that history taking from patients during their visit to the hospital is close to interview surveys.9 This study made such an assumption while assessing whether there is a specific symptom profile or other factors perceivable in our setting that might help alert physicians for earlier referrals, facilitating an earlier diagnosis of endometriosis.
Studies have identified the causes for the delay in referral at the levels of both the patient and the physician. Women are culturally trained to normalize gynecological symptoms such as dysmenorrhea. Modesty prevents some women from disclosing their sex-life-related symptoms such as dyspareunia and infertility. Added to these is the generally low public awareness about endometriosis and its symptoms. All these may lead to patients delaying their presentation or not divulging some key symptoms of endometriosis. At the physician’s level, the causes for the delay include low awareness, insufficient experience in diagnosing and managing endometriosis symptoms, culturally acquired tendencies to normalize gynecological symptoms, and using non-discriminatory diagnostic tools.9,13
Methods
This is a retrospective observational cohort study on women who attended the gynecology clinic at Sultan Qaboos University Hospital (SQUH) with the diagnosis of endometriosis from January 2011 to December 2019. The hospital is a tertiary care facility receiving referrals from primary and secondary healthcare institutions. Suitable subjects for the study were identified by searching the electronic medical record system of the hospital (TrackCare®) for the International classification of disease version 10 (ICD-10) for ‘endometriosis’ as a keyword. The main code is N80 for endometriosis with sub-codes N80.0 to N80.9 including ‘endometriosis in cutaneous scar’, ‘endometriosis of fallopian tube’, ‘endometriosis of ovary’, ‘endometriosis of pelvic peritoneum’, ‘endometriosis of rectovaginal septum and vagina’, ‘endometriosis of uterus’, ‘endometriosis unspecified’, and ‘other endometriosis’. The health records were reviewed to extract the required information including demographic data, symptoms at presentation, reproductive, medical, and surgical history, how the diagnosis of endometriosis was made, and the course of treatment. As ultrasound is a ubiquitous initial diagnostic tool for gynecological problems in primary and secondary health institutions in Oman, and because its findings often lead to a gynecologic referral, we decided to include ultrasound diagnosis of endometrioma as a special entity related to endometriosis profile, although it is not a symptom. Women with coexisting diagnoses of gynecological or other types of malignancy were excluded. Postmenopausal women were also excluded.
The interval from the onset of symptoms to the initiation of evaluation was not found consistently recorded in the case files. Therefore, we used the mean age at diagnosis as a surrogate indicator to mark the delay from the onset of symptoms to the final diagnosis. Based on that, the delay was assumed to be the minimum delay reported in the literature: a period of six years.23 Ethical approval for the study was granted by the Medical Research and Ethics Committee of the College of Medicine and Health Sciences at Sultan Qaboos University, No. SQU-EC/066/18, MREC#1696 dated July 1, 2018.
The collected data was statistically analyzed using the SPSS Statistics (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Descriptive statistics was used to present the sample demographic characteristics. Categorical variables were reported as frequencies and percentages and chi-square test verified their significance. A p-value ≤ 0.050 was considered significant. For continuous variables, mean and SD were used to present the data. Levens test was used to evaluate the difference in the mean when there was a significant difference in the size of the groups and when the data was not normally distributed.
Results
The preliminary search of the diagnosis of endometriosis returned 1338 visits. After removing duplicates and cases that did not meet the inclusion criteria, we selected the cases of N = 262 women with the diagnosis of endometriosis who attended the gynecology clinic in the nine-year period.
In our cohort, most endometriosis cases (198; 75.6%) were diagnosed by surgery. The rest (64; 24.4%) were diagnosed clinically based on symptoms profile, clinical examinations, and imaging studies. As the interval from the onset of symptoms to the initiation of evaluation was not consistently recorded in case files, we took the age at diagnosis as a surrogate.10 The mean age at first presentation to the SQUH clinic was 32.1±7.2 years (median = 32.0 years; range = 15–51 years). The mean age at diagnosis was 30.7±6.8 (median = 30.0 years; range = 15–51 years). Some of these women had the diagnosis made in another institution prior to their presentation to SQUH. Table 1 shows the age groups of women at diagnosis.
Table 1: Patients’ age at diagnosis (N = 262).
|
< 20
|
9
|
3.4
|
|
20–24
|
43
|
16.4
|
|
25–29
|
72
|
27.5
|
|
30–34
|
64
|
24.4
|
|
35–39
|
39
|
14.9
|
|
40–44
|
25
|
9.5
|
|
≥ 45
|
10
|
3.8
|
The reasons for referral were based on investigation findings and presenting symptoms. Ovarian cyst was present in 210 (80.2%) patients, while 166 (63.4%) reported at least one type of pain symptom (dysmenorrhea, dyspareunia, dyschezia, or chronic pelvic pain), and 119 (45.4%) reported infertility. Table 2 depicts all the reasons for referral.
Table 2: Reasons for referral for endometriosis care (in the order of prevalence) (N = 262).
|
Ovarian cyst
|
210
|
80.2
|
52
|
19.8
|
|
Dysmenorrhea
|
128
|
48.9
|
134
|
51.1
|
|
Primary infertility
|
88
|
33.6
|
174
|
66.4
|
|
Abnormal uterine bleeding
|
33
|
12.6
|
229
|
87.4
|
|
Secondary infertility
|
31
|
11.8
|
231
|
88.2
|
|
Chronic pelvic pain
|
25
|
9.5
|
237
|
90.5
|
|
Dyspareunia
|
13
|
5.0
|
249
|
95.0
|
|
Scar endometriosis
|
8
|
3.1
|
254
|
96.9
|
|
Dyschezia
|
4
|
1.5
|
258
|
98.5
|
Ultrasound identified ovarian endometrioma in 80.1% of cases, rendering it a potential early warning tool for the earlier referral. Indeed, the mean age at diagnosis was two years lower for the women whose ovarian endometrioma was found by ultrasound than who did not have such a diagnosis (30.3±6.7 years vs. 32.4±7.1 years, respectively). However, this difference was not statistically significant (p = 0.238).
The second most prevalent symptom was pain. Those who had pain were diagnosed younger (30.0±6.8 years) than those who did not have (31.6±7.0 years). Again, the difference was not statistically significant (p = 0.894; CI: -2.58–2.91). Endometriosis-related pain found in this population were dysmenorrhea, dyspareunia, dyschezia, and chronic pelvic pain. As described in Table 2, dysmenorrhea was present in 128 women (48.9%), dyspareunia in 13 (5.0%), chronic pelvic pain in 25 (9.5%), and dyschezia in four (1.5%). One type of pain symptom was reported by 112 (42%) women, while 18 (6.9%) reported two types, and 6 (2.3%) reported three types of endometriosis-related pain. Two women (0.8%) reported all four types of pain symptoms. However, the presence or absence of any of the four recorded types of pain had no significant correlation with the age at diagnosis (p = 0.134).
Most women in our sample (163; 62.2%) were married. Dyspareunia was reported by 13 of 163 (6.65%) married women with pelvic endometriosis (excluding scar and umbilical endometriosis). The mean age at diagnosis did not change whether they had one type or several types of pain (p = 0.439).
Among those who reported infertility, the mean age at diagnosis was 30.8±5.4. There was no significant difference between primary and secondary infertility and the age of diagnosis of endometriosis (p = 0.510). Among the 163 married women in the sample, 88 (54.0%) had primary infertility and 31 (19.0%) had secondary infertility. The mean age at diagnosis of endometriosis for all women with infertility was 30.3 years, with 30.0 years for those with primary infertility, and 33.3 years for those with secondary infertility. There was a low intergroup significance, one-way analysis of variance; p-value being 0.056.
The only factor that had a significant correlation with the age at diagnosis was time. The later the year of diagnosis, the earlier the patient age at diagnosis. Pearson correlation confirmed this chronological trend (p = 0.047) [Figure 1].
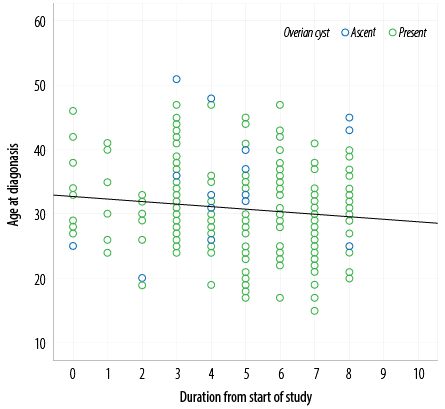 Figure 1: Relationship between the patient age at diagnosis and the calendar year of diagnosis.
Figure 1: Relationship between the patient age at diagnosis and the calendar year of diagnosis.
Discussion
This study demonstrates, using real-world clinical practice health records, that the mean age at diagnosis of endometriosis in the current sample was 30.7±6.8 years with a range of 15–51 years. The age at diagnosis based on symptoms was 30.3 years for women with endometrioma, 30.0 years for women with any type of pain symptom, and 30.3 years for women with infertility. The mean age at diagnosis of endometriosis was not impacted with statistical significance by the presence, absence, type, or multiplicity of symptoms. These results are similar to the findings in Brazilian and American populations.18,22,24 Interestingly, the Brazilian study found that patients with infertility had a shorter diagnostic delay of endometriosis compared to patients presenting with chronic pelvic pain.22
A recent study on women with similar ethnicity as our cohort was conducted by Mousa et al.11That study found the average age at diagnosis of endometriosis to be 34.04±4.81 years with an estimated delay in diagnosis of 11.6 years, which was higher than our corresponding figures (30.7±6.8 years with an assumed delay in diagnosis of about 7–8 years). The difference in our results could be attributed to various factors such as methodological differences. Theirs was a combined hospital, clinic, and population-based study on 2610 Arab ancestry women in the UAE who completed self-administered questionnaires. Although our patients were Arabs, they were all Omanis and thus relatively a homogenous group. Whereas the women in Mousa et al,11 study were of different Arabic backgrounds and different social backgrounds including Emirati nationals and expatriates hailing from many Arab countries.
Ultrasound is an important diagnostic tool in gynecology. Being low-cost and widely available, it is the first imaging method used for the evaluation of almost all gynecologic complaints including the endometriosis-related symptoms.2,25,26 Ultrasound is also used to detect the different endometriosis phenotypes such as superficial peritoneal endometriosis, ovarian endometrioma, and the deep infiltrating endometriosis (DIE).25,27 The presence of endometrioma might be a marker of more pelvic involvement with the disease, DIE, and a higher incidence of intestinal involvement.26,28,29 Diagnosing endometrioma on pelvic ultrasound is simple.27,29,30 Evidence shows that endometrioma is present in 20–70% of patients with endometriosis and that ultrasound has 70–90% sensitivity and 60–98% specificity to detect it.25,26,31 By that evidence, a case of endometriosis diagnosed by ultrasound should lead to an earlier referral for evaluation and hence an earlier diagnosis. However, this was not shown to be true in our study. Despite endometrioma being diagnosed by ultrasound in 75.6% of our study population, that did not result in a statistically significant earlier diagnosis compared to those who did not have an ultrasound-based diagnosis of endometrioma.
A common assumption related to pain is that the presentation of more than one type of pain symptom (dysmenorrhea, dyspareunia, chronic pelvic pain, and dyspareunia in any mix) should alert the physician towards an earlier referral, leading to an earlier diagnosis. This too was also not shown to be a valid assumption.
The only studied factor that significantly influenced the age at diagnosis was the timeline of the study. During the nine-year period covered by the study (January 2011 to December 2019) the diagnoses were progressively being made at earlier ages [Figure 1]. This is in accordance with other studies from the USA which reported the diagnostic delay as shortening over time.32 However, a study from the UK found the diagnostic delay as remaining unchanged over two decades.23 Several explanations can be offered for the earlier diagnosis from patients, community, and medical sides. Rising public awareness of the symptoms and the nature of the disease may have prompted patients to request for early referrals leading to reduced diagnostic delay. Additional reasons may be better awareness among the primary health care physicians, increased availability of ultrasound facilities, and updated guidelines for management.
As our study utilized real-world medical records, it has the limitation of lacking reliable information on the duration of symptoms. This was probably because the accurate estimate of onset of symptoms was either not enquired about or not recorded or was memory biased. Therefore, this study used the mean age at diagnosis as surrogate for delay in diagnosis. Another issue was that the endometriosis-related pain symptoms could have been underestimated by some physicians (a tendency also reported elsewhere),23 adding to the likelihood of under-reporting of frequency of the symptoms, especially dyspareunia.
The study shows that endometriosis patients, irrespective of the differences in their clinical profiles and symptoms, tend to have similar mean age at diagnosis, indicating that no specific symptoms predict an earlier diagnosis. However, the gradual shortening of the mean age at diagnosis over the nine-year period is a positive trend. It is important to study the factors resulting in early diagnoses of endometriosis to implement effective strategies to decrease the diagnostic delay and so avoid the negative consequences of such delays.
Conclusion
Based on this study, there is no specific symptom profile that might help make the diagnosis of endometriosis earlier to decrease women’s suffering. However, over time referrals are being made earlier, likely due to increasing awareness of women and their physicians.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004 Oct;160(8):784-796.
- 2. Giudice LC. Clinical practice endometriosis. N Engl J Med 2010 Jun;362(25):2389-2398.
- 3. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Prim 2018;4(1):1-25.
- 4. Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol 2019 Apr;220(4):354.e1-354.e12.
- 5. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep 2017 Mar;6(1):34-41.
- 6. Johnatty SE, Stewart CJ, Smith D, Nguyen A, O’ Dwyer J, O’Mara TA, et al. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci Reports 2020;10:3621.
- 7. Nezhat C, Li A, Abed S, Balassiano E, Soliemannjad R, Nezhat A, et al. Strong association between endometriosis and symptomatic leiomyomas. JSLS J 2016;20(3).
- 8. Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis–prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod 2005 Aug;20(8):2309-2316.
- 9. Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod 2012 Dec;27(12):3412-3416.
- 10. El-Maraghy M. The impact of endometriosis symptoms on health related quality of life and work productivity in Egypt. Austin J Obstet Gynecol 2017;4(3):1078-1086.
- 11. Mousa M, Al-Jefout M, Alsafar H, Becker CM, Zondervan KT, Rahmioglu N. Impact of endometriosis in women of Arab ancestry on: health-related quality of life, work productivity, and diagnostic delay. Front Glob women’s Heal 2021:66.
- 12. Ballard K, Lowton K, Wright J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril 2006 Nov;86(5):1296-1301.
- 13. Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women’s lives: a qualitative study. BMC Womens Health 2014 Oct;14(123):123.
- 14. Surrey E, Soliman AM, Trenz H, Blauer-Peterson C, Sluis A. Impact of endometriosis diagnostic delays on healthcare resource utilization and costs. Adv Ther 2020;37(3):1087-1099.
- 15. Vanhie A, Fassbender A, O D, Tomassetti C, Meuleman C, Peeraer K, et al. How to develop an electronic clinical endometriosis research file integrated in clinical practice. Biomed Res Int 2015;2015:460925.
- 16. Bogner K, Landrock U. Response biases in standardised surveys. GESIS Surv Guidel; 2016.
- 17. Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand 2003 Jul;82(7):649-653.
- 18. Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod 1996 Apr;11(4):878-880.
- 19. Brinkman LN, Saeed MS, Beck AF, Ponti-Zins MC, Unaka NI, Burkhardt MC, et al. Sociodemographic characteristics and patient and family experience survey response biases. Patient Exp J 2021 Aug;8(2):18-25.
- 20. Singh S, Soliman AM, Rahal Y, Robert C, Defoy I, Nisbet P, et al. Prevalence, symptomatic burden, and diagnosis of endometriosis in Canada: cross-sectional survey of 30 000 women. J Obstet Gynaecol Can 2020 jul;42(7):829-838.
- 21. Aday LA, Chiu GY, Andersen R. Methodological issues in health care surveys of the Spanish heritage population. American Journal of Public Health 1980 Apr;70(4):367-374.
- 22. Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL; MS A. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod 2003 Apr;18(4):756-759.
- 23. Ghai V, Jan H, Shakir F, Haines P, Kent A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J Obstet Gynaecol 2020 Jan;40(1):83-89.
- 24. Stratton P. The tangled web of reasons for the delay in diagnosis of endometriosis in women with chronic pelvic pain: will the suffering end? Fertil Steril 2006 Nov;86(5):1302-1304.
- 25. Hoyos LR, Johnson S, Puscheck E. Endometriosis and imaging. Clin Obstet Gynecol 2017 Sep;60(3):503-516.
- 26. Piessens S. Is it time to include assessment of the most common gynaecological condition in the routine ultrasound evaluation of the pelvis? Australas J Ultrasound Med 2019 May;22(2):83-85.
- 27. Van Holsbeke C, Van Calster B, Guerriero S, Savelli L, Paladini D, Lissoni AA, et al. Endometriomas: their ultrasound characteristics. Ultrasound Obstet Gynecol 2010 Jun;35(6):730-740.
- 28. Guerriero S, Ajossa S, Minguez JA, Jurado M, Mais V, Melis GB, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2015 Nov;46(5):534-545.
- 29. Chapron C, Pietin-Vialle C, Borghese B, Davy E, Foulot HE, Chopin N. ENDOMETRIOSIS Associated ovarian endometrioma is a marker for greater severity of deeply infiltrating endometriosis. Fertility and Sterility 2009 Aug 1;92(2):453-457.
- 30. Gowri V, Al Shukri M, Al Khaduri M, Machado L. Clinical and histological profile of surgically managed benign adnexal masses. Oman Med J 2014 May;29(3):239-241.
- 31. Exacoustos C, De Felice G, Pizzo A, Morosetti G, Lazzeri L, Centini G, et al. Isolated ovarian endometrioma: a history between myth and reality. J Minim Invasive Gynecol 2018 Jul - Aug;25(5):884-891.
- 32. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt) 2017 Jul;26(7):788-797.