Superior mediastinal syndrome (SMS) is a term that refers to a combination of superior vena cava syndrome (SVCS), which is compression of the SVC with flow obstruction, along with compression of the trachea.1,2 In children, SMS is more commonly caused by non-Hodgkin’s lymphoma (NHL) and T-cell acute lymphoblastic leukemia (T-cell ALL). The signs and symptoms of SMS are mainly due to the mass effect on vital structures causing airway obstruction, tamponade, and cardiogenic shock, all of which can be fatal. Therefore, prompt diagnosis through meticulous history-taking and clinical examination is essential.
Case report
A six-year-old previously healthy boy presented to a secondary hospital with a history of choking and cyanosis two weeks prior, which was followed by intermittent coughing, difficulty in breathing, stridor, dysphagia, and low-grade intermittent fever. His breathing difficulty was worsened on lying down. On examination, his heart rate was 146 beats/minute, respiratory rate 38 breaths/minute, blood pressure 116/60 mmHg, oxygen saturation 100% in room air, and temperature of 37 oC. He had facial congestion, periorbital and neck swelling, stridor, and decreased air entry on the right side of the chest. His complete blood count (CBC) and differentials were normal and his chest radiograph (CXR) was reported as normal. He was diagnosed with foreign body aspiration (FBA) and planned for bronchoscopy. During anesthesia, the patient developed hypotension and desaturation followed by hypoxic bradycardic arrest and required cardiopulmonary resuscitation with return of spontaneous circulation at three minutes. A subsequent attempt with flexible bronchoscopy resulted in a similar episode so the procedure was aborted; however, tracheal compression was noted. He was kept intubated and in the lateral position to alleviate desaturation after which he was transferred to Sultan Qaboos University Hospital, a tertiary hospital, where he was admitted to the pediatric intensive care unit (PICU) for further evaluation and management.
During transport, he had episodes of desaturation in supine position which resolved when the head of the bed was kept at 45 degrees. In the PICU, his vital signs were similar but with 96% oxygen saturation at FiO2 0.6. His face was plethoric, edematous with raised jugular venous pressure, and he had swelling of the neck and upper chest. The examination also revealed cervical lymphadenopathy and hepatomegaly. He had decreased air entry on the right side of the chest anteriorly. The rest of the systemic examination was unremarkable. Investigation done showed a normal CBC and peripheral smear. The anteroposterior view of CXR showed widened mediastinum involving the paratracheal strip and no evidence of atelectasis, unilateral hyperinflation, or cardiomegaly. Chest computed tomography revealed anterior and superior mediastinal mass compressing the SVC, with narrowing of lumen of the ascending aorta, transverse arch, and descending aorta, as well as obliteration of the main bronchus and its branches [Figure 1]. Echocardiography showed a large soft tissue mass in the upper mediastinum encasing the SVC and ascending aorta causing obstruction to the SVC and flow acceleration in the aorta and transverse arch.
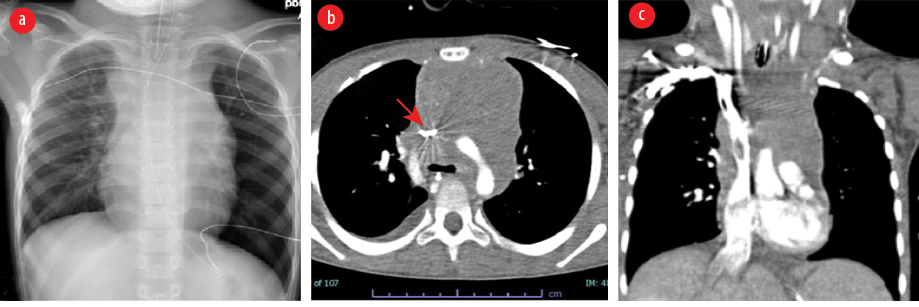 Figure 1: Imaging before treatment. (a) Chest X-ray showing a large mediastinal mass in the region of the superior mediastinum and the patient was intubated. (b) CT showing a large soft tissue density mass lesion and is resulting in compression of the superior vena cava and was slit-like on the axial image (red arrow). (c) CT coronal reformation redemonstrating a large soft tissue density mediastinal mass.
Figure 1: Imaging before treatment. (a) Chest X-ray showing a large mediastinal mass in the region of the superior mediastinum and the patient was intubated. (b) CT showing a large soft tissue density mass lesion and is resulting in compression of the superior vena cava and was slit-like on the axial image (red arrow). (c) CT coronal reformation redemonstrating a large soft tissue density mediastinal mass.
The patient was kept intubated and ventilated in a moderate setting. A central venous line was inserted in the right femoral vein and intravenous cannulation in his upper extremities was avoided. Morphine infusion was started for analgesia and sedation, avoiding bolus doses as well as the use of muscle relaxants. He was started on high-dose methylprednisolone as a life-saving measure to alleviate the fatal complications of SMS. The patient was taken to the operating theater for an excisional biopsy. However, due to persistent desaturation on initiation of anesthesia, the procedure was aborted. Based on the result of the bone marrow aspiration and trephine biopsy, he was diagnosed with T-cell ALL. He was started on standard chemotherapy protocol including monitoring and management of tumor lysis syndrome (TLS) during the induction phase of chemotherapy.
During the course of management, he showed dramatic improvement in the clinical features of SMS and was successfully extubated on day eight of admission and transferred out of PICU to the hematology/oncology ward. Repeat CXR was normal and follow-up chest CT showed normal caliber unobstructed SVC, aorta, main bronchus, and its branches [Figure 2]. He was discharged home clinically well and followed-up in the hematology/oncology outpatient clinic.
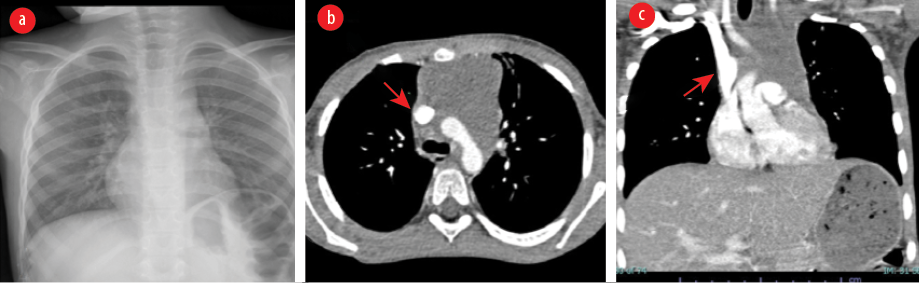 Figure 2: Imaging after treatment. (a) Chest X-ray showing reduced size of the superior mediastinal mass and endotracheal tube was removed. (b) CT showing reduction in the size of soft tissue density mass lesion in the superior mediastinum. There was a significant improvement in earlier seen compression of SVC and now showing better caliber on the axial image (red arrow). (c) CT coronal reformation showing reduction in the size of earlier seen mediastinal mass.
Figure 2: Imaging after treatment. (a) Chest X-ray showing reduced size of the superior mediastinal mass and endotracheal tube was removed. (b) CT showing reduction in the size of soft tissue density mass lesion in the superior mediastinum. There was a significant improvement in earlier seen compression of SVC and now showing better caliber on the axial image (red arrow). (c) CT coronal reformation showing reduction in the size of earlier seen mediastinal mass.
Discussion
SMS is a life-threatening pediatric oncologic emergency caused by the coexistence of the signs and symptoms of SVC obstruction and compression of the trachea and/or bronchi leading to airway compromise.1–3 The SVC receives blood from the upper part of the body and accounts for 35% of venous drainage to the heart.2 Obstruction of SVC causes facial plethora, cyanosis, swelling of face and upper body, jugular vein fullness, engorgement of collateral veins, conjunctival suffusions, and low cardiac output leading to hypotension.1–4 Impeded venous drainage to the brain leads to cerebral edema manifested by visual changes, cognitive disturbances, syncope, seizures, and altered sensorium.1–3 Respiratory symptoms include cough, dyspnea, orthopnea, stridor, and hoarseness.1,2 It can also cause dysphagia secondary to compression of the esophagus.
Children with anterior mediastinal masses (AMM) are more prone to SMS due to their small thoracic cavity, compressible airway cartilage, and little reserve with high oxygen consumption. One study reported that 27.6% of children with AMM developed SMS, of which 72.1% of cases were diagnosed with T-cell ALL.5
Our patient initially presented to a secondary hospital with classical signs and symptoms of SMS; however, misled with a history of coughing and choking on food followed by stridor and difficulty of breathing (which is also in keeping with SMS). The other signs of SMS including facial plethora, distended veins of neck, and upper chest swelling were disregarded and the more common diagnosis of FBA was pursued leading to an almost fatal complication during bronchoscopy.
In such cases, it is important to consider sub-specialty consult and multidisciplinary approach before proceeding to invasive procedures due to their associated life-threatening complications. Therefore, a high level of clinical suspicion and awareness of the presence of the pediatric SMS is of prime importance in early detection and timely management. Nasir et al reported that patients with SMS who required mechanical ventilation had a mortality of 100%.5
Diagnosis of SMS is based on classical clinical findings while laboratory tests are helpful in identifying the underlying etiology. Diagnostic workup includes CBC with peripheral smear, flow-cytometry, tumor markers, bone marrow aspirate and trephine biopsy, lymph node biopsy, CXR, CT chest, and echocardiography imaging studies.2,6 During sedation, particularly before intubation, it is important to remember that anesthesia may lead to increase venous capacitance, decreasing preload that would in turn acutely reduce cardiac output and may lead to hypotension and cardiac arrest. In addition, SVC occlusion and pulmonary artery compression may lead to cardiovascular collapse with a substantial risk of death. The use of muscle relaxants takes away the patient’s natural and physiological ability to stent their airways. Endotracheal intubation may not necessarily alleviate airway obstruction if the mass compression on the airway is distal to the endotracheal tube. The diagnostic workup should be tailored according to the clinical scenario with selection of the least invasive procedure and is best performed under local anesthesia wherever possible.2. If necessary, use of inhalational gases in a controlled environment with backup extracorporeal membrane oxygenation is advised.7–10
The goals of therapy are to relieve mass effect that leads to compression of vital structures (heart, major vessels, and major airways) as well as treatment of underlying etiology. Emergency management is of crucial importance including proper positioning of patient, elevation of the head and neck at a 45-degree or prone position, or if awake, in the position preferred, fluid boluses to promote adequate preload, avoiding sedation and in particular muscle relaxants to prevent sudden airway collapse, the most feared complication. Avoid intubation and mechanical ventilation unless necessary and if required, to be done in a controlled environment with extracorporeal membrane oxygenation back-up. Intravenous access in the upper extremities is avoided as it augments SVC obstructions.11 Corticosteroids are used acutely for debulking of mediastinal mass that compromises vital structures leading to airway or hemodynamic compromise. If administered, monitoring and management of TLS are essential.6,12 Our TLS protocol includes sending laboratory studies for uric acid, calcium, phosphate, potassium, urea, and creatinine every eight hours. Prophylaxis with allopurinol 10–20 mg/Kg/day, starts before induction and continues throughout the first week.
In our patient, there was no peripheral blast cell with the presence of SMS. However, these findings should not stop the clinician from doing further investigations to delineate etiology. Bone marrow aspiration and trephine biopsy were done and showed T-cell ALL after histopathological confirmation. Therefore, chemotherapy was initiated which yielded an excellent outcome. As previously emphasized, definitive treatment of the underlying etiology is of extreme significance.
Conclusion
SMS is an acute life-threatening emergency that requires prompt identification and timely management. A high index of suspicion is of paramount importance. The most common causes of SMS in the pediatric age group are hematological malignancies. Prognosis depends upon the underlying disease and response to treatment, and is usually excellent for chemo-sensitive tumors, as in the featured case.
Disclosure
The authors declared no conflicts of interest. Parental consent was obtained.
Acknowledgments
The authors would like to thank Ms. Sheila Magpayo, Sultan Qaboos University Hospital-Department of Child Health, for her help in editing this report.
references
- 1. Gupta V, Ambati SR, Pant P, Bhatia B. Superior vena cava syndrome in children. Indian J Hematol Blood Transfus 2008 Mar;24(1):28-30.
- 2. Jain R, Bansal D, Marwaha RK, Singhi S. Superior mediastinal syndrome: emergency management. Indian J Pediatr 2013 Jan;80(1):55-59.
- 3. Ozcan A, Unal E, Karakukcu M, Coskun A, Ozdemir MA, Patiroglu T. Vena cava superior syndrome in the children with mediastinal tumors: single-center experience. North Clin Istanb 2020 Apr;7(3):255-259.
- 4. Marwaha RK, Kulkarni KP. Superior vena cava obstruction in childhood acute lymphoblastic leukemia. Indian Pediatr 2011 Jan;48(1):78-79.
- 5. Nasir S, Jabbar R, Rehman F, Khalid M, Khan MR, Haque A. Morbidity and mortality associated with pediatric critical mediastinal mass syndrome. Cureus 2020 Jun;12(6):e8838.
- 6. Roy M, Bandyopadhyay R, Pandit N, Sengupta S. Superior Vena Cava Syndrome: A presenting feature of mediastinal germ cell tumor. OMJ 2010;25:131-133.
- 7. Chaudhary K, Gupta A, Wadhawan S, Jain D, Bhadoria P. Anesthetic management of superior vena cava syndrome due to anterior mediastinal mass. J Anaesthesiol Clin Pharmacol 2012 Apr;28(2):242-246.
- 8. Gothard JW. Anesthetic considerations for patients with anterior mediastinal masses. Anesthesiol Clin 2008 Jun;26(2):305-314, vi.
- 9. Sharifian Attar A, Jalaeian Taghaddomi R, Bagheri R. Anesthetic management of patients with anterior mediastinal masses undergoing chamberlain procedure (anterior mediastinostomy). Iran Red Crescent Med J 2013 Apr;15(4):373-374.
- 10. Williams A, Singh G, George SP. Procedural sedation for a child with a mediastinal mass and superior vena caval syndrome. J Anaesthesiol Clin Pharmacol 2015 Jul-Sep;31(3):421-424.
- 11. Arya LS, Narain S, Tomar S, Thavaraj V, Dawar R, Bhargawa M. Superior vena cava syndrome. Indian J Pediatr 2002 Apr;69(4):293-297.
- 12. Kumari I, Gupta S, Singhal PP. Superior vena caval syndrome in children–a case report. Middle East J Anaesthesiol 2006 Jun;18(5):933-938.