|
Abstract
Objectives: The purpose of the present study was to determine the alterations in high-sensitivity C-reactive protein and Tumor Necrosis factor alpha levels in the blood serum of pseudoexfoliation syndrome cases (a disease with similar risk factors as systemic endothelial dysfunction diseases) and to compare the results with healthy individuals.
Methods: High-sensitivity C-reactive protein and Tumor Necrosis factor alpha levels were determined in 30 cases with pseudoexfoliation syndrome and in 30 control patients of the same age and sex, by enzyme-linked immunosorbent assay.
Results: The levels of high- sensitivity C-reactive protein and Tumor Necrosis factor alpha in the blood serum of patients with pseudoexfoliation syndrome (3.95±0.88 mg/l, 3.32±0.99 pg/ml, respectively) were significantly higher than in the control group (2.51±0.79mg/l, 0.43±0.15 pg/ml, respectively) p=0.001, p=0.002.
Conclusion: The results suggest that increased levels of high- sensitivity C-reactive protein and Tumor Necrosis factor alpha, as markers of inflammation and peripheral endothelial dysfunction in pseudoexfoliation syndrome, may be risk factors for systemic and ocular manifestations of pseudoexfoliation syndrome.
Keywords: Pseudoexfoliation syndrome; Tumor necrosis factor alpha; High sensitivity C-reactive protein.
Introduction
Pseudoexfoliation syndrome (PEX) is a common age related elastic fibrillopathy, leading to an accumulation of characteristic abnormal fibrillar material, not only in ocular tissues, but in skin and connective tissue or visceral organs, in different intra- and extra ocular tissues. Although the syndrome is recognized to be a systemic disorder; it is the most common identifiable cause of secondary open angle glaucoma. PEX is associated with excessive synthesis and deposition of an abnormal elastic microfibrillar material in all tissues of the anterior segment of the eyes, anterior vitreous face and systemic tissues. Excessive production and accumulation of abnormal matrix components may be due to increased de novo synthesis, decreased turnover of matrix components, or both.1 A possible mechanism for the development of PEX and its subsequent progression to glaucoma may be the combination of clogging up of the trabeculum by pseudoexfoliation materials and/or pigments released from the iris, as well as trabecular endothelial dysfunction.2,3 A significant correlation between PEX, cerebrovascular and systemic cardiovascular disease has been shown.4
Vascular dysregulation, blood flow disturbances, and elevated plasma homocysteine levels are found to be associated with PEX.4,5 High-sensitivity C-reactive protein (hsCRP) is both a marker and a regulatory protein of many inflammatory pathways.6 This protein is a known predictor of vascular atherosclerotic events such as coronary artery disease, atherothrombosis, and peripheral vascular disease; and it may have a role in the pathogenesis of endothelial dysfunction and pathogenesis of atherosclerosis. Elevated plasma hsCRP levels have been reported in patients with non-arteritic anterior ischemic optic neuropathy and normal tension glaucoma a while ago.7,8
Both matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) expression are affected by tumor necrosis factor alpha (TNF-α). MMP mediated extracellular matrix turnover and TIMPs regulate the activity of MMPs. An increased level of TNF-α might induce extra MMPs and/or TIMPs.9,10
We have postulated a possible role for hsCRP and TNF-α in the pathophysiology of systemic endothelial dysfunction, vascular blood flow disturbances and insufficient matrix degradation in PEX. In this study, the systemic hsCRP and TNF-α levels as markers of inflammation and peripheral endothelial dysfunction were eveluated in PEX patients and were compared with a control group.
Methods
This prospective cross sectional study was performed on 30 patients with PEX and 30 control subjects in Nikokari Eye Hospital, Tabriz, Iran.
Ethical approval was obtained from the Medical Ethics Committee of Tabriz University of Medical Sciences and written informed consent was received from all patients according to the tenets of the Declaration of Helsinki.
All enrolled patients were scheduled for cataract surgery and underwent a complete ophthalmic examination, including tonometric assessment of intraocular pressure (IOP), measurement of cup to disc ratio (CDR), and slit lamp biomicroscopy. The patients were evaluated by slit lamp biomicroscopy for the presence of exfoliative material along the pupillary border and on the iris without dilating the eye. After pupil dilation, the patients were also analyzed for the presence of white material deposits on the anterior lens surface. Additionally, in the patients with PEX, gonioscopy and standard automated perimetry (SAP) were performed with a Humphrey field analyzer HFA II (Carl Zeiss Meditec Inc., Dublin, CA) using a 30-2 threshold program with a standard Swedish interactive threshold algorithm (SITA) strategy.
PEX was diagnosed if clinical examination revealed deposition of pseudoexfoliative material on the anterior lens capsule or at the pupillary border, the presence of transillumination defects near the pupil accompanied by normal optic nerve head finding, as well as visual field and an IOP less than 21 mmHg.
Control subjects had IOP less than 22 mmHg, normal visual fields, no evidence of glaucomatous changes in optic disc, and no pseudoexfoliative material at the anterior lens capsule or pupillary margin. All the patients with a history of trauma, inflammatory or infectious disorders, migraine, Raynaud's phenomenon, diabetes, ischemic heart disease, hypertension, hypercholesterolemia, renal and hepatic dysfunction, cerebral vascular accident, immune deficiency and treatment with corticosteroids or nonsteroidal anti-inflammatory drugs, antihypertensives, cholesterol lowering, aspirin, nitrates, hormone replacement and antioxidants were excluded.
All samples were obtained from the patients through a peripheral vein after 12 hour fasting. The subsequent serums were separated within 30 minutes and the samples were kept frozen at -70°C until the analysis was done.
hsCRP was measured using a commercial quantitative ELISA kit (Monobind Inc company) and TNF-α concentration was measured using a sandwich Elisa kit (Bender MedSystems GmbH, Vienna, Austria,) which has an inter assay co-efficient of variations of 2.8-5.7% and a lower limit of detection 7.8 pg/ml.
The data was expressed as mean±standard deviation. All the statistical analysis were performed using SPSS statistical software (statistical product and services solutions, version 16.0, SPSS Inc, Chicago, IL). The t test and Mann-Whitney U test were used as deemed appropriate (after checking data using Kolmogorov-smirnov Z test) for statistical analyses.
Results
All of the patients completed the study and none were excluded. All measurements were performed successfully, without any failure in determining the TNF-α levels owning to insufficient sampling or any other complication during the analysis. Demographic and blood sampling data for the study groups are shown in Table 1.
There were no statistically significant differences between the groups in terms of age, sex distribution, body mass index, systolic and diastolic blood pressure and lipid profile. The hsCRP and TNF-α levels in PEX samples were 3.95±0.88 mg/l and 3.32±0.99 pg/ml and 2.51±0.74 mg/l and 0.43±0.15 pg/ml in the control samples, respectively. We observed a significant increase in hsCRP and TNF-α levels in the PEX samples compared to the controls (p=0.001, p=0.002).
Table 1: Demographic and sampling data in the study groups.
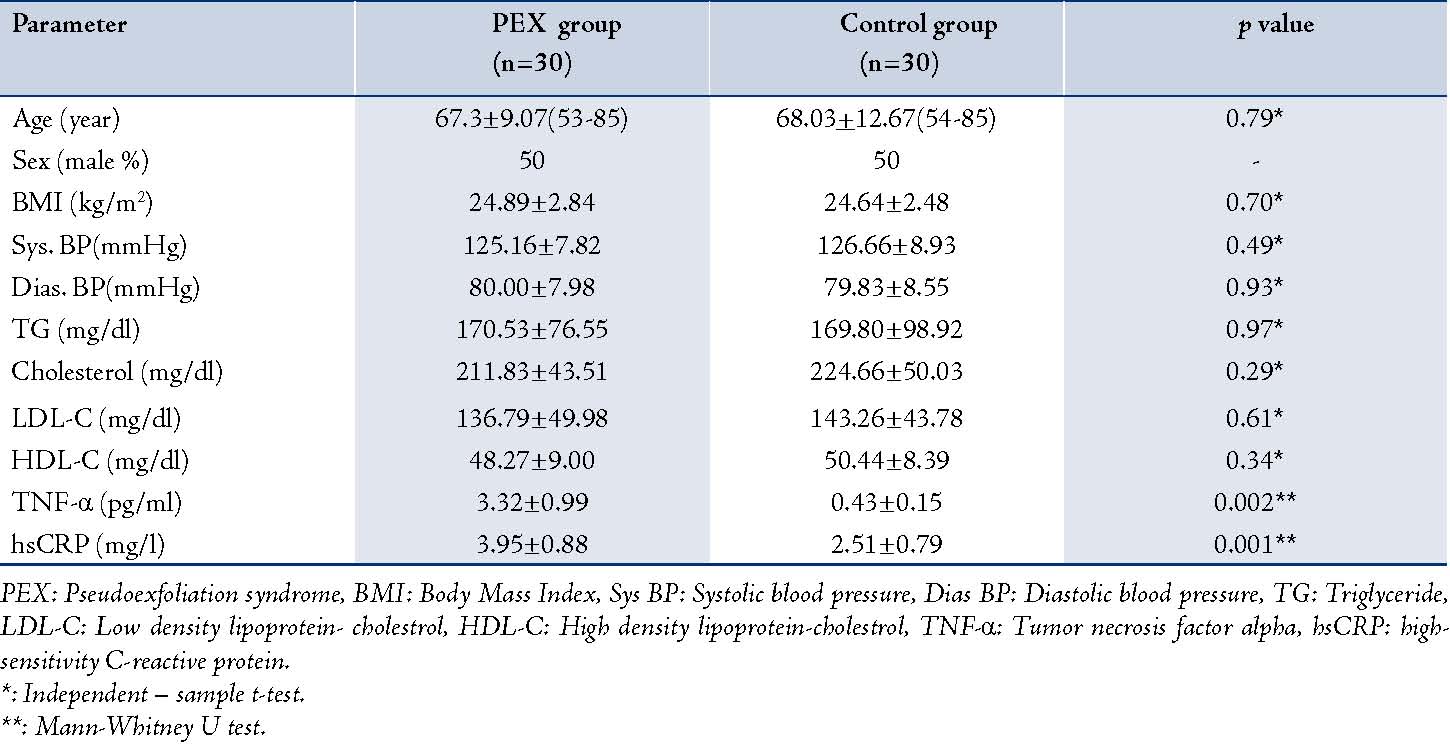
PEX: Pseudoexfoliation syndrome, BMI: Body Mass Index, Sys BP: Systolic blood pressure, Dias BP: Diastolic blood pressure, TG: Triglyceride, LDL-C: Low density lipoprotein- cholestrol, HDL-C: High density lipoprotein-cholestrol, TNF-α: Tumor necrosis factor alpha, hsCRP: high-sensitivity C-reactive protein.
*: Independent – sample t-test.
**: Mann-Whitney U test.
Discussion
PEX represents a complex, multifactorial and late-onset disease involving both genetic and non genetic factors in its etiopathogenesis. There is increasing evidence that cellular stress conditions, such as oxidative stress and ischemia/hypoxia constitute the major mechanisms involved in the pathophysiology of PEX and glaucoma.11,12
Earlier studies have reported that elevated homocysteine levels are determined in plasma, aqueous humor, and tear fluid in patients with PEX.13,14 The elevated homocysteine stimulates inflammatory cytokines such as TNF-α and markers such as C-reactive protein (CRP).15 Elevated levels of homocysteine in PEX patients with and without glaucoma may partly explain the increased risk of vascular disease among patients with PEX.4,13,14
Yuksel et al. reported that CRP is not a predictive marker of inflammation and peripheral endothelial dysfunction in PEX; however the results of our study showed a statistically significant increase in hsCRP levels in PEX.16 In contrast, our results reflected that hsCRP as a marker of inflammation may show the existing peripheral endothelial dysfunction in PEX. Yuksel et al. in their study excluded all disease that can affect CRP levels except severe hypertension, so the conflicting results of their study may be due to this selection bias. But in the current study, we strictly excluded all conditions which may affect the levels of inflammatory biomarkers, thus the results are more reliable.16
Some evidence suggests an association between PEX and systemic endothelial dysfunction, as well as vascular blood flow disturbances.3,17,18 Atalar et al. showed that systemic endothelial function is impaired in PEX patients using high-resolution ultrasound.19 CRP provokes an inflammatory reaction in the vascular endothelium and smooth muscle, thus elevated CRP levels were associated with vascular endothelial dysfunction and plaque formation.5 CRP induces adhesion molecule expression in human endothelial cells and this response is mainly arranged by proinflammatory cytokines.20
In this study, there was a significant difference in hsCRP levels between the study groups. Therefore, we thought that hsCRP may play a role in PEX or may be a reliable marker of blood flow disturbances in PEX in the absence of systemic cardiovascular disease.
TNF-α is a ubiquitous cytokine in various physiologic, as well as pathologic processes like inflammation, immunoregulation, proliferation and apoptosis. During the last few years, several immunological components have been shown to be involved in the pathogenesis of various types of glaucoma.21,22 TNF-α is considered to be a neuroprotective component of the immune system, which mediates the expression of a wide range of genes essential for neuronal survival. Contrary to its neuroprotective role, TNF-α can also serve as a neurodegenerative factor that induces the mitochondria-mediated apoptotic pathway.23,24 Thus, a delicate balance between the two pathways determines the survival of the cell, and any shift in equilibrium may have deleterious effects.
Previous studies have shown the relationships between several ocular disease and increased TNF-α levels. Diabetic patients with retinopathy have increased serum levels of TNF-α, which may be predictive of retinopathy in all diabetic patients.25,26 Sugita et al. detected significantly elevated levels of TNF-α in the ocular fluid of patients with active uveitis.27 Sawada et al. have shown that TNF-α levels in aqueous humor were significantly higher in glaucoma patients than cataract patients, particularly in PEX G.28 Corderio et al. found that a few weeks after certain pressure load conditions were applied, TNF-α was expressed, which was followed by nerve degeneration. The expression of intraocular TNF-α is considered to be the result of the secretion from ocular resident cells including iris, ciliary body, and retinal ganglion cells.29,30
The data of a prior study showed that TNF-α concentrations in vitreous humor are higher than those in the aqueous humor, which suggests that some proportion of TNF-α in the aqueous humor is the result of diffusion from the vitreous humor.27 We evaluated plasma levels of TNF-α in the PEX patients without glaucoma and we found that TNF-α levels in the PEX patients were significantly higher than in the control group. To the best of our knowledge, this is the first study to measure the serum levels of TNF-α in PEX patients in comparison with a control group.
We measured serum levels of the patients' proinflammatory cytokines in order to evaluate the role of systemic neuroinflammatory processes in the pathogenesis of this syndrome, similar to other inflammatory diseases which have already been studied.31 It was concluded that the elevated systemic levels of this marker even before progression to glaucoma may contribute to the ocular and extra ocular findings of this syndrome.
The fact that in this study we only measured two markers in PEX patients is one of the limitations of the study, but the obtained results may be the basis for further studies on many other cytokines which may be involved in the pathogenesis of this syndrome.
Conclusion
Overall, this study suggests that hsCRP is a predictive marker of inflammation and peripheral endothelial dysfunction; while TNF-α is a proinflammatory cytokine, and both may be involved in the pathogenesis of PEX. To our knowledge, this is the first report of serum TNF-α levels in PEX patients. However, further studies are necessary to elucidate the clinical role of inflammatory cytokines in the pathophysiology of PEX and to establish an exact correlation between PEX and systemic inflammation.
Acknowledgements
This work was supported by a grant from the vice-chancellor for research of Tabriz University of Medical Sciences, Tabriz, Iran. We express our appreciation to all the patients who took part in the study.
References
1. Ritch R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 2003 May;22(3):253-275.
2. Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol 2006 May;141(5):921-937.
3. Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol 2001 Jan-Feb;45(4):265-315.
4. Bleich S, Roedl J, Von Ahsen N, Schlötzer-Schrehardt U, Reulbach U, Beck G, et al. Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Ophthalmol 2004 Jul;138(1):162-164.
5. Altintaş O, Maral H, Yüksel N, Karabaş VL, Dillioğlugil MO, Cağlar Y. Homocysteine and nitric oxide levels in plasma of patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 2005 Jul;243(7):677-683.
6. Torres JL, Ridker PM. Clinical use of high sensitivity C-reactive protein for the prediction of adverse cardiovascular events. Curr Opin Cardiol 2003 Nov;18(6):471-478.
7. Kesler A, Irge D, Rogowski O, Bornstein N, Berliner S, Shapira I, et al. High-sensitivity C- reactive protein measurements in patients with non-arteritic anterior ischemic optic neuropathy: a clue to the presence of a microinflammatory response. Acta Ophthalmol (Copenh) 2009 Mar;87(2):216-221 .
8. Su WW, Ho WJ, Cheng ST, Chang SH, Wu SC. Systemic high-sensitivity C-reactive protein levels in normal-tension glaucoma and primary open-angle glaucoma. J Glaucoma 2007 May;16(3):320-323.
9. Schlötzer-Schrehardt U, Lommatzsch J, Küchle M, Konstas AG, Naumann GO. Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2003 Mar;44(3):1117-1125.
10. Meijer MJ, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Hommes DW, Verspaget HW. Role of matrix metalloproteinase, tissue inhibitor of metalloproteinase and tumor necrosis factor-α single nucleotide gene polymorphisms in inflammatory bowel disease. World J Gastroenterol 2007 Jun;13(21):2960-2966.
11. Sorkhabi R, Ghorbanihaghjo A, Ahoor MH. Oxidative stress in psudoexfoliation syndrome. Indian J Ophthalmol 2011;23:27-32.
12. Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis 2011;17:41-46.
13. Roedl JB, Bleich S, Reulbach U, Rejdak R, Kornhuber J, Kruse FE, et al. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma 2007 Mar;16(2):234-239.
14. Puustjärvi T, Blomster H, Kontkanen M, Punnonen K, Teräsvirta M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 2004 Sep;242(9):749-754.
15. Holven KB, Aukrust P, Retterstol K, Hagve TA, Mørkrid L, Ose L, et al. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand J Clin Lab Invest 2006;66(1):45-54.
16. Yüksel N, Pirhan D, Altintaş O, Cağlar Y. Systemic high-sensitivity C-reactive protein level in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Glaucoma 2010 Aug;19(6):373-376.
17. Visontai Z, Merisch B, Kollai M, Holló G. Increase of carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol 2006 May;90(5):563-567.
18. Yüksel N, Anik Y, Kiliç A, Karabaş V, Demirci A, Cağlar Y. Cerebrovascular blood flow velocities in pseudoexfoliation. Graefes Arch Clin Exp Ophthalmol 2006 Mar;244(3):316-321.
19. Atalar PT, Atalar E, Kilic H, Abbasoglu OE, Ozer N, Aksöyek S, et al. Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int Heart J 2006 Jan;47(1):77-84.
20. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000 Oct;102(18):2165-2168.
21. Funayama T, Ishikawa K, Ohtake Y, Tanino T, Kurosaka D, Kimura I, et al. Variants in optineurin gene and their association with tumor necrosis factor-alpha polymorphisms in Japanese patients with glaucoma. Invest Ophthalmol Vis Sci. 2004 Dec;45(12):4359-67 4567.
22. Joachim SC, Wuenschig D, Pfeiffer N, Grus FH. IgG antibody patterns in aqueous humor of patients with primary open angle glaucoma and pseudoexfoliation glaucoma. Mol Vis 2007;13:1573-1579.
23. Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem 2004 Jul;279(31):32869-32881.
24. Tezel G, Yang X. Comparative gene array analysis of TNF-alpha-induced MAPK and NF-kappaB signaling pathways between retinal ganglion cells and glial cells. Exp Eye Res 2005 Aug;81(2):207-217.
25. Zorena K, Myśliwska J, Myśliwiec M, Balcerska A, Hak Ł, Lipowski P, et al. Serum TNF-alpha level predicts nonproliferative diabetic retinopathy in children. Mediators Inflamm. 2007;2007:92196.
26. Doganay S, Evereklioglu C, Er H, Türköz Y, Sevinç A, Mehmet N, et al. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002 Mar;16(2):163-170.
27. Sugita S, Takase H, Taguchi C, Mochizuki M. The role of soluble TNF receptors for TNF-alpha in uveitis. Invest Ophthalmol Vis Sci 2007 Jul;48(7):3246-3252.
28. Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci 2010 Feb;51(2):903-906.
29. Cordeiro MF, Guo L, Luong V, Harding G, Wang W, Jones HE, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A 2004 Sep;101(36):13352-13356.
30. Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 2006 Dec;26(49):12633-12641.
31. Shukla RK, Kant S, Bhattacharya S, Mittal B. Association of cytokine gene polymorphisms in patients with chronic obstructive pulmonary disease. Oman Med J 2012 Jul;27(4):285-290.
|