Initially, COVID-19 was considered predominantly a respiratory infection, with respiratory failure leading to mortality.1 Since then, various extrapulmonary manifestations of the disease have been described. Multi-organ involvement has been confirmed by the detection of viral RNA in tissues other than the respiratory epithelium.2,3 A common extrapulmonary manifestation of COVID-19 is liver injury.4,5 A meta-analysis of studies on liver injury in COVID-19 showed elevated alanine aminotransferase (ALT) in an average of 18% (range = 4–40%) cases, aspartate aminotransferase (AST) in 21% (range = 4–53%) cases, total bilirubin in 6% (range = 1–18%) cases, and decreased albumin in 6% (range = 3–11%) cases.5 Hypertransaminasemia with COVID-19 is associated with more likelihood of admission to the intensive care unit (ICU), the need for mechanical ventilation (MV), and in-hospital mortality.6–10
Severe COVID-19 is also suggested to be a risk factor for developing acute kidney injury (AKI) with a poor prognosis that includes in-hospital mortality.11,12,13 Some AKI patients require renal replacement therapy, significantly increasing the cost of their treatment. Thus, knowing the risk factors for AKI associated with COVID-19 may help avoid the most negative outcomes by enabling early identification of patients who may benefit from aggressive therapy, frequent monitoring, and periodic adjustments in treatment.
Previously, there have been reports of possible association between liver and kidney injuries in COVID-19.8,14 According to Piano et al,8 the prevalence of AKI was 22% and 13% in the group with and without abnormal liver function test results (p = 0.009). Huang et al,15 reported higher serum creatinine (sCr) levels (88.5 vs. 70 mmol/L; p = 0.020) in patients with ischemic hepatitis compared with patients without it.
The origin of the relationship between liver and kidney injuries in COVID-19 patients is not yet clear due to limited data. Therefore, we aimed to investigate clinical features of elevated transaminases on admission in patients with COVID-19, its association with the risks of AKI, and poor outcomes including mortality.
Methods
For this single-center retrospective analysis, the subjects were recruited from the patients admitted with COVID-19 from 13 April to 20 June 2020 at Moscow City Hospital. The study was approved by the ethics committee of Peoples' Friendship University of Russia Moscow (No. 07-20). Being a retrospective study, informed consent from the patients was not required. We included patients > 18 years of age with laboratory-confirmed COVID-19 and whose AST and ALT levels were assessed within 48 hours of hospitalization. Readmission, acute surgical pathology, duration of hospitalization < 48 hours, history of chronic liver disease, history of continuous renal replacement therapy, and single measurement of sCr per hospitalization were the exclusion criteria.
All selected patients underwent an imaging investigation, a chest computer tomography (CT) scan, and standard clinical laboratory tests (including complete blood count, urinalysis, and blood chemistry test) on admission and in dynamics.
Abnormality in aminotransferases was defined as ALT > 40 U/L and/or AST > 40 U/L as specified in local laboratory documents. The definition and staging of AKI were based on kidney disease: improving global outcomes 2012 criteria.16 Baseline sCr was determined as the minimum sCr value during hospitalization, or if available, the last sCr in the previous six months before hospitalization. Patients admitted to the hospital with AKI or those who developed AKI during the first 48 hours were classified as having community-acquired AKI (CA-AKI). Hospital-acquired AKI (HA-AKI) was defined as any AKI documented after 48 hours of hospital admission. Hematuria was defined as the presence of more than three erythrocytes in the field of view and proteinuria as > 0.3 g/L. We assessed the lung injury by chest CT and categorized the degrees of lung injury in four stages as follows: stage 1: < 25% lung injury; stage 2: 26–50%; stage 3: 51–75%; and stage 4: > 75%. The term acute decompensated heart failure (ADHF) was used to describe cases with previous history of chronic stable heart failure with the typical symptoms and signs of decompensation of heart failure during hospitalization.
Mathematical and statistical analyses were performed using Stata software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.). Continuous variables were described as mean and SD (mean±SD) for normally distributed data or median and interquartile range (IQR) values for non-normal distribution. Qualitative parameters were presented as frequencies and percentages. The means of continuous variables were compared using independent t-tests. The ratios of qualitative variables were compared using the chi-squared test. Multinomial logistic regression was performed to explore the potential predictors of CA-AKI and HA-AKI. Covariates in the model included variables at admission: age > 65 years, sex, hypertension, coronary artery disease, ADHF, history of chronic kidney disease (CKD), diabetes mellitus, history of cancer, anemia (hemoglobin < 120 g/L in females and < 130 g/L in males), white blood cells < 4 × 109/L (leukopenia), lymphocytes < 1.2 × 109/L (lymphopenia), thrombocytes < 150 × 109/L (thrombocytopenia), and oxygen saturation < 93% on admission with room air breathing, C-reactive protein > 75 g/L, serum albumin < 35 g/L, total bilirubin > 21 μmol/L, AST > 40 U/L, ALT > 40 U/L, hematuria, and proteinuria. Relative risk ratios (RRRs) were calculated with 95% CIs. P-value of < 0.050 was considered statistically significant.
Results
Out of the total 1204 patients hospitalized with COVID-19, we excluded 376 and finally selected 828 patients as the study subjects [Figure 1]. Their mean age was 65.0±16.0 years; 51.4% were male.
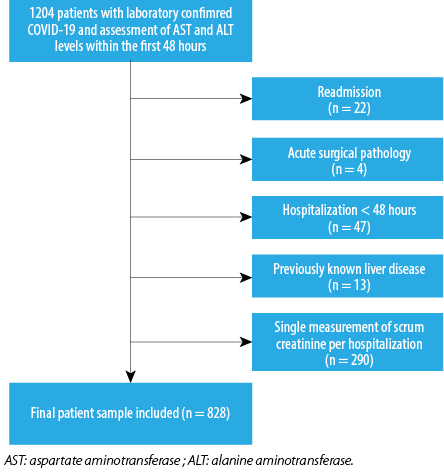 Figure 1: The selection process of the study population.
Figure 1: The selection process of the study population.
Anthropometric data on admission was available for 513 patients. Within 48 hours of admission, urinalysis was performed in 772 patients, and lactate dehydrogenase, ferritin, and D-dimer were measured in 308, 263, and 624 patients, respectively.
The most prevalent comorbidity found in patients was hypertension (70.3%) followed by obesity (51.9%). Nearly half (49.0%) had decreased oxygen saturation levels (< 93%) when breathing ambient air. Elevated aminotransferase levels on admission were noted in 40.8% of patients. The mean length of hospital stay was 12 (9–15) days, while 25.1% of patients spent at least one day in the ICU. The mean length of ICU stay was 5 (2–9) days. Hematuria on admission was observed in 124 of 772 (16.1%) patients, and proteinuria in 254 of 772 (32.9%) patients. In-hospital mortality occurred to 172 out of the total 828 (20.8%) patients [Table 1].
Table 1: Clinical characteristics of study patients.
|
Age, mean ± SD
|
65.0 ± 16.0
|
630 ± 16.0
|
66.0 ± 16.0
|
0.006
|
|
Male, n (%)
|
426 (51.4)
|
156 (46.2)
|
270 (55.1)
|
0.010
|
|
Day of illness at admission, Me (IQR)
|
6 (3–8)
|
6 (3–8)
|
5 (3–8)
|
0.070
|
|
Comorbidities, n (%)
|
|
|
|
|
|
Hypertension
|
582 (70.3)
|
232 (68.6)
|
350 (71.4)
|
0.400
|
|
Diabetes mellitus
|
215 (26.0)
|
87 (25.7)
|
128 (26.1)
|
0.900
|
|
CAD
|
141 (17.0)
|
54 (16.0)
|
87 (17.8)
|
0.500
|
|
Malignancy
|
84 (10.1)
|
30 (8.9)
|
54 (11.0)
|
0.300
|
|
CKD
|
70 (8.5)
|
24 (7.1)
|
46 (9.4)
|
0.200
|
|
ADHF
|
120 (14.5)
|
48 (14.2)
|
72 (14.7)
|
0.800
|
|
SBP, mmHg, mean ± SD
|
126.0 ± 16.0
|
126.0 ± 16.0
|
126.0 ± 16.0
|
0.900
|
|
DBP, mmHg, mean ± SD
|
80.0 ± 10.0
|
80.0 ± 11.0
|
80.0 ± 10.0
|
0.800
|
|
SatO2 on room air, Me (IQR)
|
94 (92–95)
|
93 (91–94)
|
94 (92–95)
|
< 0.001
|
|
Laboratory parameters
|
|
|
|
|
|
AST, U/L, Me (IQR)
|
34 (25–50)
|
55 (44–72)
|
26 (21–32)
|
-
|
|
ALT, U/L, Me (IQR)
|
24 (17–40)
|
44 (32–64)
|
18 (14–24)
|
-
|
|
sCr, mmol/L, Me (IQR)
|
91 (77–114)
|
93 (77–119)
|
89 (76–112)
|
0.040
|
|
WBC ×109/L, Me (IQR)
|
6.1 (4.4–8.3)
|
6.2 (4.5–8.8)
|
6 (4.4–8.1)
|
0.200
|
|
WBC < 4 × 109/L, n (%)
|
142 (17.1)
|
54 (16.0)
|
88 (18.0)
|
0.500
|
|
Lymphocytes, × 109/L, Me (IQR)
|
1 (0.7–1.4)
|
1 (0.7–1.3)
|
1 (0.7–1.4)
|
0.700
|
|
Lymphocytes < 1.2 × 109/L, n (%)
|
506 (61.1)
|
207 (61.2)
|
299 (6.01)
|
0.900
|
|
Platelets × 109/L, Me (IQR)
|
191 (151–252)
|
195 (153–247)
|
191 (148–256)
|
0.900
|
|
Platelets < 150 × 109/L, n (%)
|
204 (24.6)
|
77 (22.8)
|
127 (25.9)
|
0.300
|
|
Hb, g/L, Me (IQR)
|
130 (119–144)
|
133 (122–146)
|
129 (117–142)
|
0.002
|
|
Anemia, n (%)
|
216 (26.1)
|
75 (22.2)
|
141 (28.8)
|
0.030
|
|
C-reactive protein, mg/L, Me (IQR)
|
74 (28–125)
|
91 (37–146)
|
65 (24–109)
|
< 0.001
|
|
LDH, U/L, Me (IQR)
|
361 (265–539)
|
454 (333–660)
|
301 (234–412)
|
< 0.001
|
|
Total bilirubin, mmol/L, Me (IQR)
|
10 (7.3–13.8)
|
11 (8–15)
|
9.5 (7–13)
|
< 0.001
|
|
Ferritin, μg/L, Me (IQR)
|
495 (253–676)
|
627 (404–747)
|
420 (192–618)
|
< 0.001
|
|
Albumin, g/L, Me (IQR)
|
34 (31–370
|
34 (30–37)
|
34 (31–38)
|
0.300
|
|
D-dimer, μg/L, Me (IQR)
|
311 (164–593)
|
325 (192–592)
|
293 (147–593)
|
0.020
|
|
Fibrinogen, g/L, Me (IQR)
|
5.9 (4.9–6.9)
|
6 (5–7.1)
|
5.8 (4.8–6.8)
|
0.200
|
|
AKI, n (%)
|
224 (27.1)
|
110 (32.5)
|
114 (23.3)
|
0.003
|
|
CA-AKI, n (%)
|
129 (15.6)
|
71 (21.0)
|
58 (11.8)
|
< 0.001
|
*We compared groups with elevated and normal transaminases.
AST: aspartate transaminase; ALT: alanine transaminase; Me: median; IQR: interquartile range; CAD: coronary artery disease, CKD: chronic kidney disease; ADHF: acute decompensated heart failure; SBP: systolic blood pressure; DBP: diastolic blood pressure; SatO2: pulse oxygen saturation; sCr: serum creatinine; WBC: white blood cell; Hb: hemoglobin; LDH: lactate dehydrogenase; AKI: acute kidney injury; CA-AKI: community-acquired acute kidney injury; HA-AKI: hospital-acquired acute kidney injury.
Cases with elevated AST (n = 304; 36.7%) were more common than those with elevated ALT (n = 208; 25.1%). There were no differences in concomitant pathology and obesity (53.6% vs. 49.8%; p = 0.400) in the groups with and without abnormal levels of transaminases, respectively. The frequency of statins and antibacterial drugs intake before hospitalization also did not differ (10% vs. 12%, p = 0.400; 49% vs. 47%; p = 0.600, respectively). Patients with elevated transaminases on admission were more likely to be younger, female, and to have higher levels of inflammatory markers and D-dimer. The length of stay was similar between the groups (11 (9–15) and 12 (9–16) days; p = 0.400). Patients with hypertransaminasemia on admission more often had proteinuria in the first 24 hours of hospitalization (41.5% vs. 27.1%; p < 0.001), and the frequency of hematuria was nearly the same (15.4% vs. 16.5%, p = 0.700). AKI incidence was higher in patients with elevated AST and/or ALT levels on admission. There was a greater degree of lung injury as revealed by chest CT in patients with abnormal transaminase level [Figure 2].
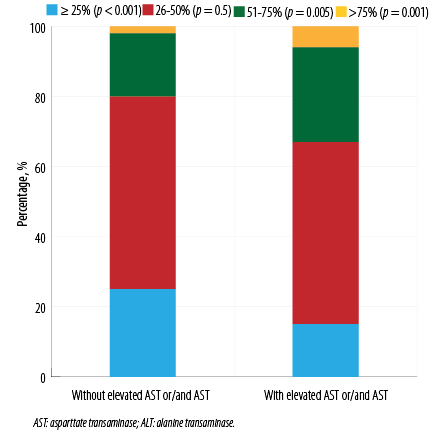 Figure 2: Levels of lung injury in patients at admission as revealed by chest CT scan, stratified by ALT and/or AST.
Figure 2: Levels of lung injury in patients at admission as revealed by chest CT scan, stratified by ALT and/or AST.
Patients with elevated ALT and/or AST on admission were more likely to be admitted to ICU and to require MV. AKI rates and in-hospital mortality were also higher among them [Figure 3]. However, there was no significant impact of elevated ALT or AST on the incidence of thrombosis.
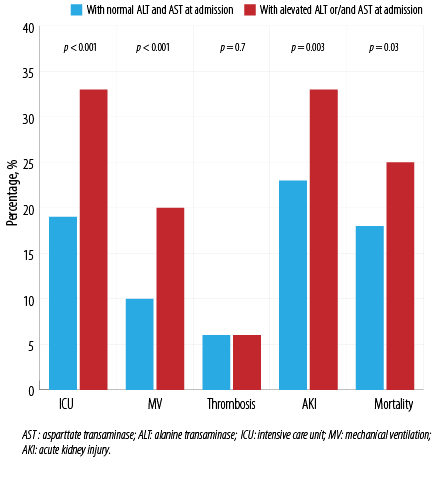 Figure 3: Outcomes in patients, stratified by ALT or AST at admission.
Figure 3: Outcomes in patients, stratified by ALT or AST at admission.
Further, the analysis was performed with division into the groups with predominant elevated ALT and AST. Patients with predominant elevated AST on admission had significantly worse outcomes, such as transfer to the ICU, need for MV, progression of lung injury by CT, mortality, and AKI incidence [Figure 4].
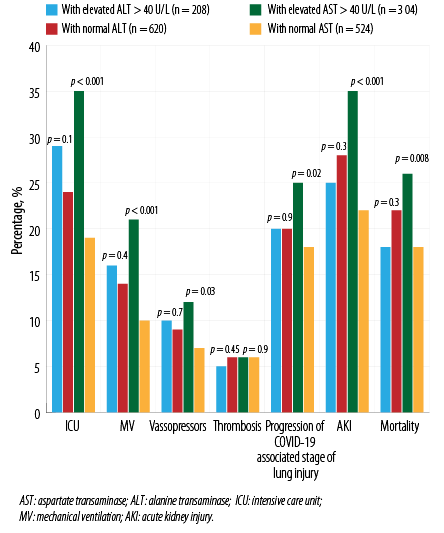 Figure 4: Outcomes in patients, stratified by elevated levels of either ALT or AST at admission.
Figure 4: Outcomes in patients, stratified by elevated levels of either ALT or AST at admission.
Hypertension, history of CKD, elevated AST, and hematuria on admission were independently associated with a significant risk of CA-AKI according to multinomial logistic regression. Age > 65 years, history of hypertension, malignancy, elevated AST, and hematuria on admission were predictors for HA-AKI [Table 2].
Table 2: Independent predictors of community-acquired acute kidney injury (CA-AKI) and hospital-acquired acute kidney injury (HA-AKI) as indicated by multinomial logistic regression.
|
Age ≥ 65 years
|
1.40
|
0.82–2.39
|
0.200
|
3.09
|
1.52–6.29
|
0.002
|
|
Sex
|
0.89
|
0.56–1.41
|
0.600
|
0.98
|
0.58–1.67
|
0.900
|
|
ADHF
|
0.99
|
0.54–1.83
|
0.900
|
1.76
|
0.95–3.28
|
0.070
|
|
CAD
|
1.43
|
0.82–2.49
|
0.200
|
0.62
|
0.31–1.22
|
0.200
|
|
Hypertension
|
2.65
|
1.38–5.01
|
0.003
|
2.66
|
1.14–6.19
|
0.020
|
|
CKD
|
2.55
|
1.32–4.92
|
0.006
|
1.84
|
0.86–3.96
|
0.600
|
|
Malignancy
|
1.73
|
0.88–3.39
|
0.100
|
2.24
|
1.09–4.56
|
0.030
|
|
Diabetes mellitus
|
1.26
|
0.78–2.04
|
0.300
|
1.24
|
0.71–2.16
|
0.400
|
|
SatO2 < 93% on room air
|
1.24
|
0.79–1.95
|
0.400
|
1.29
|
0.76–2.16
|
0.300
|
|
Anemia
|
1.14
|
0.68–1.91
|
0.600
|
1.64
|
0.94–2.87
|
0.080
|
|
Leukopenia
|
0.53
|
0.27–1.04
|
0.060
|
0.82
|
0.40–1.66
|
0.600
|
|
Lymphopenia
|
1.32
|
0.80–2.17
|
0.300
|
1.52
|
0.82–2.84
|
0.200
|
|
Thrombocytopenia
|
1.63
|
0.97–2.75
|
0.070
|
1.45
|
0.79–2.64
|
0.300
|
|
C-reactive protein > 75 g/L
|
0.96
|
0.60–1.55
|
0.900
|
1.63
|
0.93–2.86
|
0.090
|
|
AST > 40 U/L
|
2.51
|
1.46–4.31
|
0.001
|
2.13
|
1.17–3.88
|
0.010
|
|
ALT > 40 U/L
|
0.90
|
0.50–1.65
|
0.700
|
0.38
|
0.17–0.85
|
0.020
|
|
Total bilirubin > 21 mmol/L
|
1.25
|
0.56–2.81
|
0.600
|
1.83
|
0.78–4.34
|
0.200
|
|
Albumin < 35 g/L
|
1.35
|
0.82–2.21
|
0.200
|
0.86
|
0.48–1.55
|
0.600
|
|
Hematuria
|
2.57
|
1.51–4.39
|
0.001
|
2.86
|
1.59–5.16
|
0.001
|
RRR: relative risk ratio; ADHF: acute decompensated heart failure; CAD: coronary artery disease; CKD: chronic kidney disease; SatO2: pulse oxygen saturation; AST: aspartate transaminase; ALT: alanine transaminase.
However, abnormal ALT level on admission was not associated with the incidence of CA-AKI and HA-AKI in this population.
Discussion
Our study comprising 828 hospitalized patients with laboratory-confirmed COVID-19 and pneumonia showed that elevated transaminase levels were common and associated with disease severity, which is consistent with the results of recent studies.7–9 Among these, a systematic meta-analysis of 24 studies comprising 12 882 hospitalized COVID-19 patients demonstrated a high prevalence of elevated AST (40.8%) and ALT (29%). This meta analysis found that abnormal AST (OR = 2.98, 95% CI: 2.35–3.77; p < 0.001) and, to a lesser extent, ALT (OR = 1.85, 95% CI: 1.49–2.29; p < 0.001) were associated with poor outcomes, such as hospitalization in the ICU, fall in oxygen saturation below 90%, initiation of MV, or in-hospital mortality.7 A multicenter study from China found elevated AST in 48.5% and ALT in 42% of hospitalized patients, and a greater like- lihood of hypertransaminasemia in patients with higher levels of inflammatory biomarkers and severe course of COVID-19, which correlates with our findings.17 Clearly, the association of hypertransaminasemia with inflammatory markers of COVID-19 suggests their involvement in disease severity and poorer outcomes.
In our work elevated AST or ALT did not correlate with comorbidities or age, contrary to the findings of another study.8 Pathogenesis of elevated transaminases in COVID-19 is complex and not fully understood. It can potentially include several factors such as ischemic damage, as patients with elevated transaminase levels had lower saturation, damage due to systemic inflammation similar to sepsis, as well as coagulation disorders including micro- and macrovascular thrombosis.18,19
The possible relationship between hypercoagulation and hypertransaminasemia is discussed in the work of Hamadé et al,20 which retrospectively shows a potential association between elevated transaminases and venous thrombosis. Our study found no similar associations. The probable reason for the low detection rate of thrombosis in our study population was that lower extremity ultrasound was not performed routinely in all our patients, but only for those with clinical signs of thrombosis or a significant increase in D-dimer. On the other hand, Hamadé et al,20 studied only 46 patients against our 828, giving our results greater statistical power.
Earlier studies showed that elevated AST than ALT is more common in patients with COVID-19, which correlates with our findings.4,7,14,21,22 We also found that patients with elevated AST had poorer prognoses than those with elevated ALT. In a retrospective study of 2073 hospitalized COVID-19 patients from China, among all liver parameters, only elevated AST and direct bilirubin levels were associated with in-hospital mortality with adjusted hazard ratio = 1.61 (95% CI: 1.20–2.15; p = 0.001) and adjusted hazard ratio = 1.57 (95% CI: 1.14–2.16; p = 0.006), respectively.4 Also, in a meta-analysis of 64 studies comprising 11 245 COVID-19 patients, elevated AST was more common in severe diseases (45.5% for AST vs. 15% for ALT).22 Despite a large amount of data on the negative predictive value of elevated AST and the AST/ALT ratio compared to ALT in COVID-19, the reason for this difference is not completely clear and requires further investigation.
In addition, in our study, an increase in AST over 40 U/L was associated with CA-AKI (RR: 2.51; 95% CI: 1.46–4.31; p = 0.001) and was a predictor of HA-AKI (RR: 2.13; 95% CI: 1.17–3.88; p = 0.010).
The relationship between CA-AKI and elevated AST suggests a correlation with the disease severity at admission since COVID-19 patients with community-acquired AKI were admitted in more severe conditions than those without AKI.23 Also, the combination of involvement of various systems and organs reconfirms the possibility of multisystem injury in COVID-19. On the other hand, a recent study by Martínez-Rueda et al,23 showed that patients with CA-AKI with COVID-19 more frequently have comorbidities as compared with those with HA-AKI, including CKD (10% vs. 3%; p = 0.015) and hypertension (45% vs. 25%; p < 0.001), and the development of CA-AKI was associated with a history of CKD (OR = 4.17, 95% CI: 1.53–11.3), hypertension (OR = 1.55, 95% CI: 1.01–2.36), Charlson comorbidity index (OR = 1.16, 95% CI: 1.02–1.32), and the sequential organ failure assessment score (OR = 2.19, 95% CI: 1.87–2.57).The above results are consistent with ours, because in addition to elevated AST on admission, CA-AKI was statistically more common in our patients with a history of hypertension and CKD, emphasizing the higher risks of adverse outcomes for COVID-19 patients with comorbidities.
Moreover, hypertension is a recognized risk factor for renal function impairment and AKI in COVID-19 and the most common comorbidity in our study, which is supported by the literature.24–26 Parker et al,25 who aimed to investigate a possible effect of continuous anticoagulant therapy before hospitalization on the prevention of AKI, found the risk factors for AKI to be CKD, hypertension, and male sex. Our study went further and showed preexisting hypertension to be a risk factor for not only CA-AKI but also HA-AKI.
Hematuria on admission was also a predictor of both AKI forms in our study. Describing similar data, earlier studies have also demonstrated proteinuria and hematuria to be predictors of poor prognosis and HA-AKI in patients with COVID-19.27,28 The predictive role of urine changes for AKI development was known earlier.29
Before COVID-19 times, two meta-analysis showed that the prognosis in HA-AKI was worse than in CA-AKI.30,31 However, such differentiation in the context of COVID-19 is ambiguous. On the one hand, Pelayo et al,32 have shown a higher mortality rate and the need for vasopressor support in hospitalized COVID-19 patients with HA-AKI than in those with CA-AKI. On the other, Martínez-Rueda et al,23’s study on 1170 hospitalized COVID-19 patients in Mexico found both CA-AKI and HA-AKI to be associated with poor prognosis, albeit lacking statistical significance. As the emerging data are still limited and inconsistent, reliable epidemiological data on the relative prognosis of each form of AKI will take time to emerge.
Our study showed for the first time that elevated AST was a common significant factor associated with both phenotypes of AKI. An advantage of our study was the exclusion of patients with previously known liver diseases and the determination of transaminase levels on admission to the hospital, which minimized interference from other causes of cytolysis.33,34
The principal limitation of our study is its retrospective nature, as several factors might have been left out. In many patients, the sCr and transaminase levels were missed before hospital admission. Therefore, it could lead to a decreased or increased incidence of AKI and CKD. In addition, patients with preexisting undiagnosed chronic liver disease could have been inadvertently included in the study. Our definition of AKI did not include the urine volume-based criterion of < 0.5 mL/kg/h for 6 hours, which may have led to underestimation of AKI incidence. Another limitation of our study is the lack of data on the use of non-steroidal anti-inflammatory drugs (which can increase transaminase levels) by patients before hospitalization.
Further research is needed to ascertain the predictive role of hypertransaminasemia in COVID-19 patients. A comprehensive assessment of the heart function in COVID-19 patients with and without elevated AST would be useful.
Conclusion
Our study showed a high prevalence of elevated transaminases among patients hospitalized for COVID-19 in a large tertiary center in Moscow in the first half of 2020. Hypertransaminasemia at admission was associated with systemic inflammation and D-dimer level. Patients with elevated transaminases on admission had higher frequency of AKI (32.5% vs. 23.3%; p = 0.003) and greater odds for in-hospital mortality (24.6% vs. 18.2%; p = 0.030). Patients with predominantly elevated AST were more likely to have adverse outcomes than those with predominantly elevated ALT. Elevated AST above 40 U/L on admission was independently associated with the development of CA-AKI and HA-AKI. Patients with increased levels of AST at admission should be closely followed up in view of potentially poorer outcomes.
Disclosure
The authors declared no conflicts of interest. This study is supported by the Peoples' Friendship University of Russia University Strategic Academic Leadership Program Funding with local funds.
Acknowledgments
The authors are grateful to the members of Department of Internal Diseases with the Course of Cardiology and Functional Diagnostics named after Moiseev V.S., Institute of Medicine in Peoples' Friendship University of Russia) and the staff of the Therapy Department, the Intensive Care Unit of Vinogradov City Clinical Hospital (Moscow, Russia) for their cooperation and valuable efforts.
references
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al; China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020 Apr;382(18):1708-1720.
- 2. Thakur V, Ratho RK, Kumar P, Bhatia SK, Bora I, Mohi GK, et al. Multi-organ involvement in COVID-19: beyond pulmonary manifestations. J Clin Med 2021 Jan;10(3):446.
- 3. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal Tropism of SARS-CoV-2. N Engl J Med 2020 Aug;383(6):590-592.
- 4. Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, et al; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol 2021 Jun;74(6):1295-1302.
- 5. Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020 Jul;5(7):667-678.
- 6. Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, et al. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol 2020 Nov - Dec;19(6):614-621.
- 7. Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, et al. Liver disease and outcomes among COVID-19 hospitalized patients - a systematic review and meta-analysis. Ann Hepatol 2021 Mar-Apr;21:100273.
- 8. Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, et al; COVID-LIVER study group. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int 2020 Oct;40(10):2394-2406.
- 9. Lv Y, Zhao X, Wang Y, Zhu J, Ma C, Feng X, et al. Abnormal liver function tests were associated with adverse clinical outcomes: an observational cohort study of 2,912 Patients With COVID-19. Front Med (Lausanne) 2021 Jun;8:639855.
- 10. Taramasso L, Vena A, Bovis F, Portunato F, Mora S, Dentone C, et al. Higher mortality and intensive care unit admissions in COVID-19 patients with liver enzyme elevations. Microorganisms 2020 Dec;8(12):16.
- 11. Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al; Mount Sinai COVID Informatics Center (MSCIC). AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2021 Jan;32(1):151-160.
- 12. Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep 2020 Jun;5(8):1149-1160.
- 13. Chebotareva N, Berns S, Berns A, Androsova T, Lebedeva M, Moiseev S. Acute kidney injury and mortality in coronavirus disease 2019: results from a cohort study of 1,280 patients. Kidney Res Clin Pract 2021 Jun;40(2):241-249.
- 14. Hasel K, Verma S, Salim A, et al. Elevated aspartate transaminase (AST) and inflammatory markers are associated with acute kidney injury and increased mortality in hospitalized patients with COVID-19. Hepatology 2020;72(1)(SUPPL):272A.
- 15. Huang H, Li H, Chen S, Zhou X, Dai X, Wu J, et al. Prevalence and characteristics of hypoxic hepatitis in COVID-19 patients in the intensive care unit: a first retrospective study. Front Med (Lausanne) 2021 Feb;7:607206.
- 16. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1-138.
- 17. Chen F, Chen W, Chen J, Xu D, Xie W, Wang X, et al. Clinical features and risk factors of COVID-19-associated liver injury and function: a retrospective analysis of 830 cases. Ann Hepatol 2021 Mar-Apr;21:100267.
- 18. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020 Apr;382(18):1708-1720.
- 19. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020 Mar;395(10229):1054-1062.
- 20. Hamadé A, Woehl B, Talbot M, Bensalah N, Michel P, Obringer G, et al. Aminotransferases disorders associated with venous thromboembolic events in patients infected with COVID-19. Ann Hepatol 2021 Mar-Apr;21:100274.
- 21. Benedé-Ubieto R, Estévez-Vázquez O, Flores-Perojo V, Macías-Rodríguez RU, Ruiz-Margáin A, Martínez-Naves E, et al. Abnormal liver function test in patients infected with coronavirus (SARS-CoV-2): a retrospective single-center study from Spain. J Clin Med 2021 Mar;10(5):1039.
- 22. Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, et al. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol 2021 Jul;33(7):990-995.
- 23. Martínez-Rueda AJ, Álvarez RD, Méndez-Pérez RA, Fernández-Camargo DA, Gaytan-Arocha JE, Berman-Parks N, et al. Community- and hospital-acquired acute kidney injury in COVID-19: different phenotypes and dismal prognosis. Blood Purif 2021;50(6):931-941.
- 24. de Almeida DC, Franco MD, Dos Santos DR, Santos MC, Maltoni IS, Mascotte F, et al. Acute kidney injury: incidence, risk factors, and outcomes in severe COVID-19 patients. PLoS One 2021 May;16(5):e0251048.
- 25. Parker K, Hamilton P, Hanumapura P, Castelino L, Murphy M, Challiner R, et al. Chronic anticoagulation is not associated with a reduced risk of acute kidney injury in hospitalized Covid-19 patients. BMC Nephrol 2021 Jun;22(1):224.
- 26. Hachim IY, Hachim MY, Naeem KB, Hannawi H, Al Salmi I, Al-Zakwani I, et al. Kidney dysfunction among COVID-19 patients in the United Arab Emirates. Oman Med J 2021 Feb;36(1):e221.
- 27. Chaudhri I, Moffitt R, Taub E, Annadi RR, Hoai M, Bolotova O, et al. Association of proteinuria and hematuria with acute kidney injury and mortality in hospitalized patients with COVID-19. Kidney Blood Press Res 2020;45(6):1018-1032.
- 28. Nogueira SÁ, Oliveira SC, Carvalho AF, et al. Renal changes and acute kidney injury in covid-19: a systematic review. Rev Assoc Med Bras (1992). 2020;66Suppl 2(Suppl 2):112-117.
- 29. Han SS, Ahn SY, Ryu J, Baek SH, Chin HJ, Na KY, et al. Proteinuria and hematuria are associated with acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol 2014 Jun;15:93.
- 30. Huang L, Xue C, Kuai J, Ruan M, Yang B, Chen X, et al. Clinical characteristics and outcomes of community-acquired versus hospital-acquired acute kidney injury: a meta-analysis. Kidney Blood Press Res 2019;44(5):879-896.
- 31. Inokuchi R, Hara Y, Yasuda H, Itami N, Terada Y, Doi K. Differences in characteristics and outcomes between community- and hospital-acquired acute kidney injury: a systematic review and meta-analysis
. Clin Nephrol 2017 Oct;88(10):167-182.
- 32. Pelayo J, Lo KB, Bhargav R, Gul F, Peterson E, DeJoy Iii R, et al. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with covid-19 in a US inner city hospital system. Cardiorenal Med 2020;10(4):223-231.
- 33. Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol 2020 Oct;13:1756284820959183.
- 34. Harrill AH, Roach J, Fier I, Eaddy JS, Kurtz CL, Antoine DJ, et al. The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin Pharmacol Ther 2012 Aug;92(2):214-220.