HIV infection in pregnancy affects the mother, her placenta, and the fetus, with progressive viral disease altering the course of the pregnancy and resulting in perinatal or maternal morbidity and mortality.1–8 Notably, based on its natural history, HIV infection can be categorized into four stages: asymptomatic, mildly symptomatic, moderately symptomatic, and severely symptomatic (with AIDS).7,9,10 Studies have shown that HIV-positive mothers have several placental morphological changes such as greenish yellow discoloration of fetal membrane, reduced placental weight, chorioamnionitis, villitis, choriodeciduitis, funisitis, decidual cell necrosis, villous vasculopathy, etc.3,6,11–18 Studies have also shown that these mothers are at increased risk of adverse perinatal outcomes, including stillbirth, low birth weight (LBW), preterm birth, infant mortality from pneumonia or neonatal sepsis, and mother-to-child transmission of HIV.3,19–27 Importantly, Akwa Ibom state (our study location) had the second highest HIV prevalence in Nigeria, particularly among pregnant women. The prevalence of HIV in Akwa Ibom state has ranged from 4.8% to 10.9%, in contrast to the Nigerian prevalence of 4.1% since 2010.28–36
Although numerous studies have shown profound adverse histomorphometric parameters and lesions in the placenta of HIV-positive mothers with associated adverse perinatal outcomes, to the best of our knowledge, these placental histomorphometric parameters and lesions have not been surveyed or reported in Uyo, Akwa Ibom state, in general. This gap in knowledge underscores the medical importance of our study and provides an immediate rationale for it.
Therefore, in this study, we aimed to survey the range of histomorphometric parameters and lesions in the placenta of HIV-positive mothers and to relate these parameters/lesions with their stage of HIV disease in Uyo, Akwa Ibom state. Also, we aimed to compare our findings with those of other similar studies conducted elsewhere. As a deliverable, we hope that our findings will inform clinical evaluation and management of feto-maternal complications of HIV infection in affected women in Akwa Ibom state and Nigeria and help attain the third goal of the United Nations’ Sustainable Development Goals.37,38
Methods
This study was a prospective cross-sectional hospital-based analytical study involving two study groups: the test (cases) and control groups. This study was conducted at the University of Uyo Teaching Hospital (UUTH), Uyo, Akwa Ibom state, in the departments of Obstetrics and Gynecology, and Histopathology. UUTH is a 500-bed tertiary healthcare facility in the South-South region of Nigeria.
We used a convenient sampling technique. Samples (relevant historical data from case notes and their placentas) were collected from December 2015 to May 2016. All consenting HIV-positive (test group) and HIV-negative (control group) pregnant women, any time from 28 weeks of gestation before disposal of their placenta, who came to the hospital for obstetrics/delivery care within the six months of this study were included.
The exclusion criteria consisted of all non-consenting HIV-positive and HIV-negative pregnant women, all HIV-negative pregnant women with grossly abnormal placenta, such as placental tumors, and those who delivered before 28 weeks of gestation. The investigator accessed the case notes of all consenting pregnant women (HIV-positive or HIV-negative status) during the delivery period of each index subject. Important data regarding relevant historical and physical examination findings were extracted from these case notes. All collected data were recorded in the data collection form for each subject.
Histomorphometric assessment of the placenta (for both groups):
- Sample collection: at the third stage of labor, the labor ward staff takes delivery of the placenta, which is then collected by the principal investigator for labeling and placement in a wide-mouth bucket containing 10% neutral buffered formalin.
- Gross (macroscopy): the three main placental components (fetal membranes, umbilical cord, and placental disk) were examined systematically after 48 hours of fixation (consistent with the handling of an infectious specimen).2,39–44 They were weighed after examining and clots, membranes, and cords removed.2,43,45–47
- Sample section procedure:
- Umbilical cord: two cassettes.
- Fetal membrane roll: two cassettes.
- Placental disk parenchyma: six cassettes. More cassettes were submitted in cases with numerous lesions to sample all lesion types.2,39,41,42,44
- Fixation, tissue processing, embedding, microtomy, and staining were performed using standard histological techniques and stained with hematoxylin and eosin.
- Microscopy: an Olympus CX22 light microscope was used to carry out systematic histopathologic evaluations of the three components of the placenta (umbilical cord, fetal membrane, and placental disk). The terminology/criteria used for placental diagnosis in this study was according to the schemata in the studies by Huettner,2 Schwartz et al,12 and Redline et al.48–50
Ethical approval for this study was obtained from UUTH Health Research Ethics Committee (UUTH/AD/S/96/VOL.XII/115). Patient confidentiality was protected, and informed consent was obtained.
Data generated/collected in this study were written in a ‘Patient Case Report’ form and transferred into Microsoft Office Excel 2013 and SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Additionally, we calculated the placental/birth weight ratio for both groups as a ratio of placental weight to birth weight multiplied by 100.51,52 We used cross-tabulation, Pearson’s chi-square test, likelihood ratio, Fisher’s exact test, and linear-by-linear association to test for statistical differences between the variables of both groups. Statistical significance was set at a p-value of ≤ 0.05. The results were reported as text, tables, charts, graphs, and photomicrographs.
Results
A total of 144 mothers were admitted to this study (48 HIV-positive mothers as tests and 96 HIV-negative mothers as controls). The test group age range, mean, median, and mode were 21–38 years, 30.2±4.3, 30, and 29 years, respectively. The control group age range, mean, median, and mode were 19–41 years, 29.0±4.3, 28.5, and 28 years, respectively [Table 1]. The tests delivered 52.1% of their babies through cesarean section, while the controls delivered 66.7% through spontaneous vaginal delivery [Table 1]. The test group delivered 95.8% live births with 4.2% macerated stillbirths, while the controls delivered 94.8% live births and 4.2% fresh stillbirths [Figure 1]. The mean, median, and mode of birth weight of tests’ babies were 2.8±0.7 kg, 2.95 kg, and 3.3 kg, with 26.5% of infants having LBW, while the controls’ mean, median, and mode of birth weight were 3.1±0.6 kg, 3.2 kg, and 3.2 kg, with 9.4% of infants having LBW, p = 0.004 [Table 1].
Table 1: Frequency distribution of mothers’ age, mode of delivery, birth weight, placental weight, and placental-birth weight ratio between the test and control populations.
|
Age group, years
|
|
< 16
|
0
|
0.0
|
0
|
0.0
|
-
|
-
|
|
16–23
|
3
|
6.3
|
7
|
7.4
|
-
|
-
|
|
24–31
|
25
|
52.1
|
61
|
64.2
|
-
|
-
|
|
32–34
|
10
|
20.8
|
12
|
12.6
|
-
|
-
|
|
35–40
|
10
|
20.8
|
14
|
14.7
|
-
|
-
|
|
41–45
|
0
|
0.0
|
1
|
1.1
|
-
|
-
|
|
> 45
|
0
|
0.0
|
0
|
0.0
|
-
|
-
|
|
Total
|
48
|
100
|
95
|
100
|
-
|
-
|
|
Mode of delivery
|
|
Cesarean section
|
25
|
52.1
|
30
|
31.3
|
0.47
|
4.8
|
|
Instrumental
|
0
|
0.0
|
2
|
2.1
|
-
|
-
|
|
Vaginal
|
23
|
47.9
|
64
|
66.7
|
-
|
-
|
|
Total
|
48
|
100
|
96
|
100
|
-
|
-
|
|
Birth weight, kg
|
|
|
|
|
|
|
|
< 2.5
|
13
|
26.5
|
9
|
9.4
|
0.004
|
8.45
|
|
2.5–4.5
|
36
|
73.5
|
87
|
90.6
|
-
|
-
|
|
> 4.5
|
0
|
0.0
|
0
|
0.0
|
-
|
-
|
|
Total
|
49
|
100
|
96
|
100
|
-
|
-
|
|
Placental weight, g
|
|
250–399
|
6
|
12.5
|
0
|
0.0
|
0.003
|
17.96
|
|
400–549
|
11
|
22.9
|
12
|
12.8
|
-
|
-
|
|
550–699
|
17
|
35.4
|
36
|
38.3
|
-
|
-
|
|
700–849
|
12
|
25.0
|
34
|
36.2
|
-
|
-
|
|
850–999
|
1
|
2.1
|
9
|
9.6
|
-
|
-
|
|
> 999
|
1
|
2.1
|
3
|
3.2
|
-
|
-
|
|
Total
|
48
|
100
|
94
|
100
|
-
|
-
|
|
Placental/birth weight ratio
|
|
< 15.8
|
3
|
6.1
|
6
|
6.5
|
0.33
|
2.23
|
|
15.8–20.6
|
28
|
57.1
|
42
|
45.2
|
-
|
-
|
|
> 20.6
|
18
|
36.7
|
45
|
48.4
|
-
|
-
|
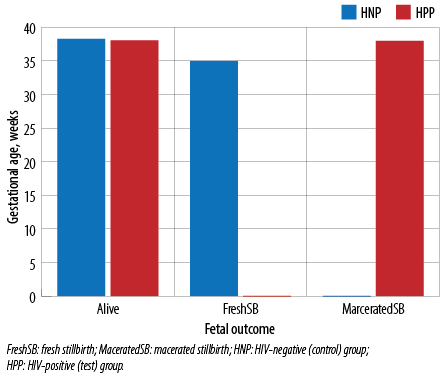 Figure 1: Frequency distribution of fetal outcome in relation to gestational age in both the test and control groups.
Figure 1: Frequency distribution of fetal outcome in relation to gestational age in both the test and control groups.
The tests’ mean, median, and mode of placental weight were 575.5±190.1 g, 550 g, and 600 g, while in the control group these values were 664.6±167.4 g, 650 g, and 700 g, respectively (p = 0.003) [Table 1]. The tests’ mean, median, and mode of placental birth weight ratio were 20.1±4.8, 19.7, and 20, and 20.54±4.57, 20.36, and 20 in the control group, respectively (p = 0.003) [Table 1].
The tests group fetal membrane displayed greenish yellow to brown discoloration in 60.4% of cases, whereas the controls displayed this discoloration in 45.3% of cases [Table 2, Figure 2]. The most common fetal membrane histopathologic lesion in the test group was acute chorioamnionitis (47.9%) and chronic choriodeciduitis (chronic inflammatory lesion involving the chorion and decidua) in the control group (72.6%); giving statistically significant p-values for acute chorio-deciduitis, chronic choriodeciduitis, decidual cell necrosis (zones of accidental cell death in the decidua), and acute chorioamnionitis [Table 2 and Figure 3].
Table 2: Placental histopathological changes and lesions in the fetal membrane in the test and control groups.
|
Greenish yellow to brown discoloration of membranes
|
29 (60.4)
|
19 (39.6)
|
43 (45.3)
|
52 (54.7)
|
0.08
|
|
Acute choriodeciduitis
|
20 (41.7)
|
28 (58.3)
|
8 (8.4)
|
87 (91.6)
|
< 0.001a
|
|
Chronic choriodeciduitis
|
13 (27.1)
|
35 (72.9)
|
69 (72.6)
|
26 (27.4)
|
< 0.001b
|
|
Acute on chronic choriodeciduitis
|
3 (6.3)
|
45 (93.8)
|
10 (10.5)
|
85 (89.5)
|
0.40
|
|
Decidual cell necrosis
|
13 (27.1)
|
35 (72.9)
|
6 (6.3)
|
89 (93.7)
|
0.001c
|
|
Chronic chorioamnionitis
|
4 (8.3)
|
44 (91.7)
|
5 (5.3)
|
90 (94.7)
|
0.47
|
|
Acute chorioamnionitis
|
23 (47.9)
|
25 (52.1)
|
20 (21.1)
|
75 (78.9)
|
0.001d
|
Note: a, b, c, and d showed statistically significant differences (p ≤ 0.05) between the test and control groups. These differences reveal a strong association with HIV infection in a, c and d, but a strong dissociation with HIV infection in b.
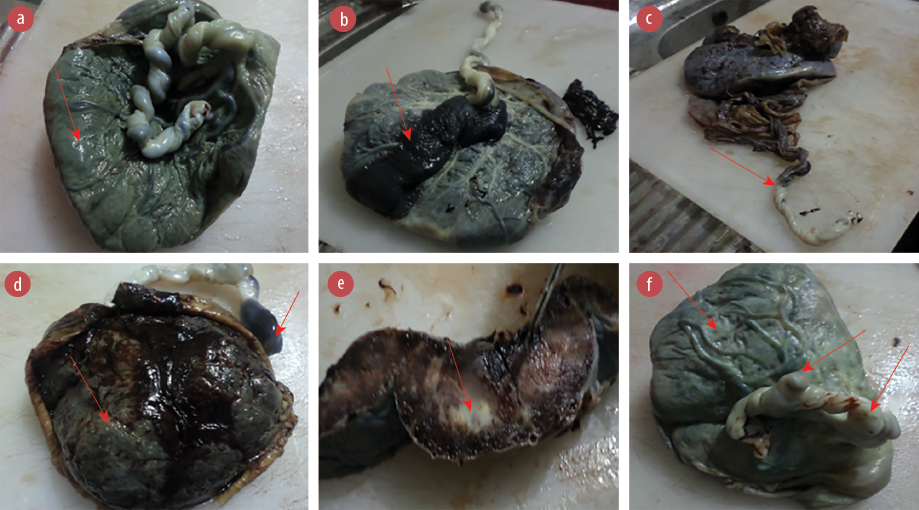 Figure 2: Gross morphology of the placentas of (a) a 36-year-old HIV-positive mother showing meconium (greenish brown) staining of the fetal surface; (b) a 30-year-old HIV-negative mother showing massive chorionic plate hematoma of the fetal surface of the placental disk; (c) a 24-year-old HIV-positive mother showing velamentous insertion of the umbilical cord to the fetal membrane instead of the fetal surface of the placental disk; (d) a 37-year-old HIV-positive mother showing massive umbilical cord hematoma as well as maternal surface of the placental disk; (e) a 19-year-old HIV-negative mother showing an area of massive infarction of the cut surface of the placental disk; and (f) a 24-year-old HIV-positive mother showing two false knots of the umbilical cord as well as opaque greenish grey maternal surface of the placental disk.
Figure 2: Gross morphology of the placentas of (a) a 36-year-old HIV-positive mother showing meconium (greenish brown) staining of the fetal surface; (b) a 30-year-old HIV-negative mother showing massive chorionic plate hematoma of the fetal surface of the placental disk; (c) a 24-year-old HIV-positive mother showing velamentous insertion of the umbilical cord to the fetal membrane instead of the fetal surface of the placental disk; (d) a 37-year-old HIV-positive mother showing massive umbilical cord hematoma as well as maternal surface of the placental disk; (e) a 19-year-old HIV-negative mother showing an area of massive infarction of the cut surface of the placental disk; and (f) a 24-year-old HIV-positive mother showing two false knots of the umbilical cord as well as opaque greenish grey maternal surface of the placental disk.
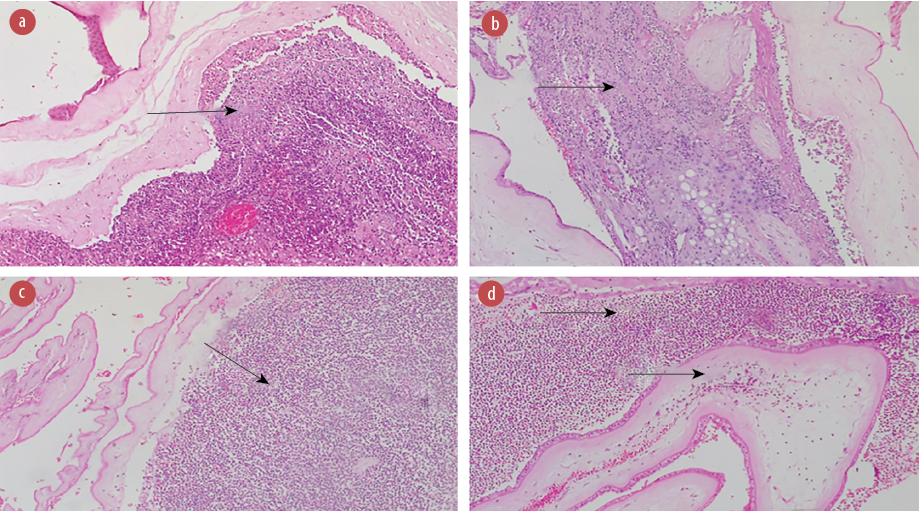 Figure 3: Photomicrographs of the fetal membrane of (a) a 34-year-old HIV-positive mother showing acute suppurative necrotizing chorioamnionitis (stage 3, grade 2) with acute choriodeciduitis; (b) a 29-year-old HIV-positive mother showing acute choriodeciduitis; (c) a 24-year-old HIV-positive mother showing acute suppurative chorioamnionitis; and (d) a 22-year-old HIV-positive mother showing severe acute suppurative deciduitis with mild acute chorionitis. All images stained with hematoxylin and eosin, magnification = 100 ×.
Figure 3: Photomicrographs of the fetal membrane of (a) a 34-year-old HIV-positive mother showing acute suppurative necrotizing chorioamnionitis (stage 3, grade 2) with acute choriodeciduitis; (b) a 29-year-old HIV-positive mother showing acute choriodeciduitis; (c) a 24-year-old HIV-positive mother showing acute suppurative chorioamnionitis; and (d) a 22-year-old HIV-positive mother showing severe acute suppurative deciduitis with mild acute chorionitis. All images stained with hematoxylin and eosin, magnification = 100 ×.
The test group’s commonest umbilical cord histopathologic lesion was acute umbilical phlebitis (14.6%) while the control group’s commonest umbilical cord histopathologic lesion was acute funisitis (5.3%) [Table 3].
Table 3: Placental histopathological lesions in the umbilical cord in the test and control groups.
|
Chronic umbilical vasculitis
|
1 (2.1)
|
47 (97.9)
|
1 (1.1)
|
94 (98.9)
|
0.62
|
|
Acute umbilical phlebitis
|
7 (14.6)
|
41 (85.4)
|
4 (4.2)
|
91 (95.8)
|
0.06
|
|
Acute umbilical panvasculitis
|
2 (4.2)
|
46 (95.8)
|
0 (0.0)
|
95 (100)
|
0.11
|
Note: there was no statistically significant difference (p ≤ 0.05) between the test and control groups. These differences reveal a weak association with HIV infection.
The test group’s commonest placental disk histopathological lesion was villous vasculopathy (33.3%) and the most common in the control group was massive perivillous fibrin deposition (38.9%); giving statistically significant p-values for chronic villitis, chronic intervillositis, acute intervillositis, maternal floor infarct, and intervillous thrombohematoma [Table 4 and Figure 4].
Table 4: Placental histopathological lesions in the placental disk to compare the test and the control groups.
|
Chronic villitis
|
0 (0.0)
|
48 (100)
|
17 (17.9)
|
78 (82.1)
|
0.002a
|
|
Chronic intervillositis
|
0 (0.0)
|
48 (100)
|
14 (14.7)
|
81 (85.3)
|
0.005b
|
|
Acute villitis
|
3 (6.3)
|
45 (93.8)
|
2 (2.1)
|
93 (97.8)
|
0.20
|
|
Acute intervillositis
|
15 (31.3)
|
33 (68.8)
|
6 (6.3)
|
89 (93.7)
|
< 0.001c
|
|
Villous vasculopathy
|
16 (33.3)
|
32 (66.7)
|
20 (21.1)
|
75 (78.9)
|
0.10
|
|
Hemorrhagic endovasculitis
|
0 (0.0)
|
48 (100)
|
0 (0.0)
|
95 (100)
|
-
|
|
Villous stromal fibrosis
|
0 (0.0)
|
48 (100)
|
1 (1.1)
|
94 (98.9)
|
1.00
|
|
Infarction
|
14 (29.2)
|
34 (70.8)
|
35 (36.8)
|
60 (63.2)
|
0.46
|
|
Cytotrophoblastic hyperplasia
|
0 (0.0)
|
48 (100)
|
0 (0.0)
|
95 (100)
|
-
|
|
Massive perivillous fibrin deposition
|
13 (27.1)
|
35 (72.9)
|
37 (38.9)
|
58 (61.1)
|
0.16
|
|
Maternal floor infarct
|
2 (4.2)
|
46 (95.8)
|
17 (17.9)
|
78 (82.1)
|
0.02d
|
|
Retroplacental hematoma
|
2 (4.2)
|
46 (95.8)
|
8 (8.4)
|
87 (91.6)
|
0.55
|
|
Acute chorionitis of chorionic plate
|
4 (8.3)
|
44 (91.7)
|
5 (5.3)
|
90 (94.7)
|
0.48
|
|
Acute chorionic plate vasculitis
|
5 (10.4)
|
43 (89.6)
|
4 (4.2)
|
91 (95.8)
|
0.28
|
Note: a, b, c, d, and e showed statistically significant differences (p ≤ 0.05) between the test and control groups. These differences reveal a strong association with HIV infection in c and e, but a strong dissociation with HIV infection in a, b, and d.
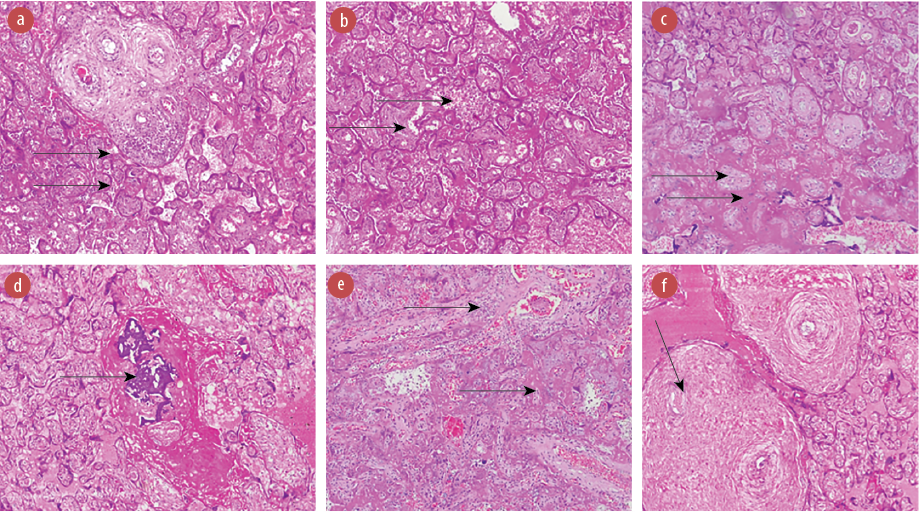 Figure 4: Photomicrographs of the placental disk of (a) a 32-year-old HIV-positive mother showing acute villitis and acute vasculitis of stem villous; (b) a 32-year-old HIV-positive mother showing infarction and acute intervillositis; (c) a 35-year-old HIV-positive mother showing infarcted villi and massive perivillous fibrin deposition;(d) a 29-year-old HIV-positive mother showing calcification and massive perivillous fibrin deposition; (e) a 30-year-old HIV-positive mother showing acute villitis and fibrin deposition; and (f) a 29-year-old HIV-positive mother showing chronic vasculitis with obliterative features.
Figure 4: Photomicrographs of the placental disk of (a) a 32-year-old HIV-positive mother showing acute villitis and acute vasculitis of stem villous; (b) a 32-year-old HIV-positive mother showing infarction and acute intervillositis; (c) a 35-year-old HIV-positive mother showing infarcted villi and massive perivillous fibrin deposition;(d) a 29-year-old HIV-positive mother showing calcification and massive perivillous fibrin deposition; (e) a 30-year-old HIV-positive mother showing acute villitis and fibrin deposition; and (f) a 29-year-old HIV-positive mother showing chronic vasculitis with obliterative features.
All images hematoxylin and eosin staining, magnification = 100 ×.
On further evaluation of these inflammatory lesions involving the fetal membrane, umbilical cord, and chorionic plate of the placenta disk, we found that the tests had the most severe forms (stage/grade) of acute chorioamnionitis and acute funisitis (20.8%), while the controls had none; giving statistically significant p-values for acute chorionitis (stage 1, grade 1), acute suppurative chorioamnionitis (stage 2, grade 1), acute suppurative necrotizing chorioamnionitis (stage 3, grade 2), acute choriodeciduitis, chronic choriodeciduitis, acute panvasculitis (stage 2, grade 1), and placental membrane inflammation (PMI) [Table 5, Figure 3 and Figure 5].
Table 5: Grading and staging of placental inflammatory lesions of the fetal membrane, umbilical cord, and chorionic plate in the test and control groups.
|
Acute chorionitis (stage 1, grade 1)
|
7 (14.6)
|
41 (85.4)
|
2 (2.1)
|
94 (97.9)
|
0.003a
|
|
Acute chorioamnionitis (stage 2, grade 1)
|
4 (8.3)
|
44 (91.7)
|
3 (3.1)
|
93 (96.9)
|
0.171
|
|
Acute suppurative chorioamnionitis (stage 2, grade 2)
|
0 (0.0)
|
48 (100)
|
12 (12.5)
|
84 (87.5)
|
0.011b
|
|
Acute suppurative necrotizing chorioamnionitis (stage 3, grade 2)
|
10 (20.83)
|
38 (79.17)
|
0 (0.0)
|
96 (100)
|
0.000c
|
|
Chronic chorioamnionitis
|
0 (0.0)
|
48 (100)
|
5 (5.2)
|
91 (94.8)
|
0.108
|
|
Acute choriodeciduitis
|
23 (47.9)
|
25 (52.1)
|
3 (3.1)
|
93 (96.9)
|
0.000d
|
|
Acute on chronic choriodeciduitis
|
3 (6.3)
|
45 (93.8)
|
8 (8.3)
|
88 (91.7)
|
0.657
|
|
Chronic choriodeciduitis
|
17 (35.4)
|
31 (64.6)
|
69 (71.9)
|
27 (28.1)
|
0.000e
|
|
Acute chorionitis of chorionic plate
|
6 (12.5)
|
42 (87.5)
|
6 (6.3)
|
90 (93.8)
|
0.201
|
|
Acute chorionic plate vasculitis
|
5 (10.4)
|
43 (89.6)
|
4 (4.2)
|
92 (95.8)
|
0.144
|
|
Acute phlebitis (stage 1, grade 1)
|
4 (8.3)
|
44 (91.7)
|
3 (3.1)
|
93 (96.9)
|
0.171
|
|
Chronic phlebitis
|
0 (0.0)
|
48 (100)
|
1 (1.0)
|
95 (99.0)
|
0.478
|
|
Acute panvasculitis (stage 2, grade 1)
|
2 (4.2)
|
46 (95.8)
|
0 (0.0)
|
96 (100)
|
0.044f
|
|
Acute funisitis (stage 3, grade 1)
|
7 (14.6)
|
41 (85.4)
|
6 (6.3)
|
90 (93.8)
|
0.099
|
|
Acute necrotizing funisitis (stage 3,
grade 2)
|
1 (2.1)
|
47 (97.9)
|
0 (0.0)
|
96 (100)
|
0.156
|
Note: a, b, c, d, e, f, and g showed statistically significant differences (p < 0.05) between the test and control groups. These differences reveal a strong association with HIV infection in a, c, d, f, and g, but a strong dissociation with HIV infection in b and e.
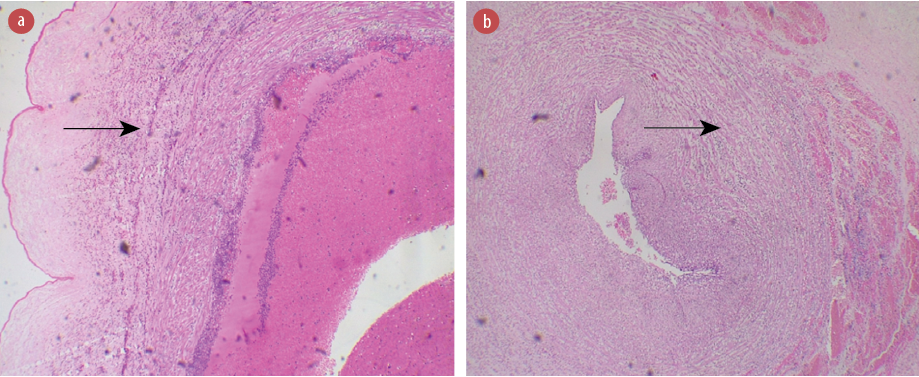 Figure 5: Photomicrographs of the umbilical cord of the placenta from (a) a 32-year-old HIV-positive mother showing acute funisitis (stage 3, grade 2). (b) A 24-year-old HIV-positive mother showing acute phlebitis. Hematoxylin and eosin staining, magnification = 100 ×.
Figure 5: Photomicrographs of the umbilical cord of the placenta from (a) a 32-year-old HIV-positive mother showing acute funisitis (stage 3, grade 2). (b) A 24-year-old HIV-positive mother showing acute phlebitis. Hematoxylin and eosin staining, magnification = 100 ×.
Importantly, the majority of the test group subjects (66.7%) were in stage 1 of HIV disease, and only two patients were in stage 4 (4.2%) [Table 6]. Both test group subjects with stage 4 HIV disease were found to have greenish yellow to brown discoloration of the fetal membrane, acute chorioamnionitis, acute choriodeciduitis, and decidual cell necrosis [Table 6]. The cross-tabulation (exploring relationships) between the stages of HIV/AIDS with mean placental/birth weight ratio was not statistically significant between the stages [Figure 6]. The test group umbilical cord lesions had acute umbilical panvasculitis strongly related to advanced HIV/AIDS disease state [Table 7]. Placental disk lesions like acute intervillositis, acute deciduitis, villous vasculopathy, and massive perivillous fibrin deposition were found commonly with advanced HIV/AIDS disease state [Table 8].
Table 6: Exploratory relationship between stages of HIV/AIDS and histopathological changes and lesions in the fetal membrane of placenta of the test group.
|
Greenish yellow to brown discoloration of membranes
|
Present (20; 62.5)
|
Present (7; 53.8)
|
Absent
|
Present (2; 100)
|
0.372
|
|
Acute chorio-deciduitis
|
Present (14; 43.8)
|
Present (4; 30.8)
|
Absent
|
Present (2; 100)
|
0.240
|
|
Chronic chorio-deciduitis
|
Present (6; 18.8)
|
Present (6; 46.2)
|
Present (1; 100)
|
Absent
|
0.073
|
|
Acute on chronic chorio-deciduitis
|
Present (3; 9.4)
|
Absent
|
Absent
|
Absent
|
0.659
|
|
Decidual cell necrosis
|
Present (8; 25.0)
|
Present (3; 23.1)
|
Absent
|
Present (2; 100)
|
0.115
|
|
Chronic chorioamnionitis
|
Present (3; 9.4)
|
Present (1; 7.7)
|
Absent
|
Absent
|
0.955
|
|
Acute chorioamnionitis
|
Present (16; 50.0)
|
Present (4; 30.8)
|
Present (1; 100)
|
Present (2; 100)
|
0.183
|
Note: there was no statistically significant difference between the stages, indicating a weak association between the lesions and the disease stages. However, the in-stage association was most significant for stage 4 disease subjects, using percentage proportionality.
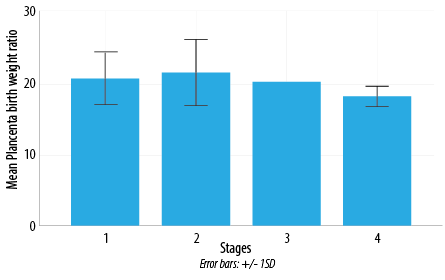 Figure 6: Exploratory relationship between stages of HIV/AIDS and mean placental/birth weight ratio in the test group. Note: F value = 0.46 and p = 0.71. There was a weak association between the stages of HIV/AIDS and the mean placental/birth weight ratios of the test group.
Figure 6: Exploratory relationship between stages of HIV/AIDS and mean placental/birth weight ratio in the test group. Note: F value = 0.46 and p = 0.71. There was a weak association between the stages of HIV/AIDS and the mean placental/birth weight ratios of the test group.
Table 7: Exploratory relationship between stages of HIV/AIDS and histopathological lesions in the umbilical cord of placenta of the test group.
|
Chronic umbilical vasculitis
|
Absent
|
Present (1; 7.7)
|
Absent
|
Absent
|
0.432
|
|
Acute umbilical phlebitis
|
Present (5; 15.6)
|
Present (2; 15.4)
|
Absent
|
Absent
|
0.909
|
|
Acute umbilical panvasculitis
|
Present (1; 3.1)
|
Absent
|
Absent
|
Present (1; 50.0)
|
0.011a
|
Note: ashowed a statistically significant difference between the stages, indicating a strong association between acute umbilical panvasculitis and the disease stages. The in-stage association for acute umbilical panvasculitis was most significant for stage 4 disease subjects, using percentage proportionality.
Table 8: Exploratory relationship between stages of HIV/AIDS and histopathological lesions in the placental disk of placenta of the test group.
|
Chronic villitis
|
Absent
|
Absent
|
Absent
|
Absent
|
-
|
|
Chronic intervillositis
|
Absent
|
Absent
|
Absent
|
Absent
|
-
|
|
Acute villitis
|
Present (2; 6.3)
|
Present (1; 7.7)
|
Absent
|
Absent
|
0.970
|
|
Acute intervillositis
|
Present (10; 31.3)
|
Present (4; 30.8)
|
Absent
|
Present (1; 50.0%)
|
0.853
|
|
Villous vasculopathy
|
Present (11; 34.4)
|
Present (4; 30.8)
|
Absent
|
Present (1; 50.0)
|
0.848
|
|
Villous stromal fibrosis
|
Absent
|
Absent
|
Absent
|
Absent
|
-
|
|
Infarction
|
Present (9; 28.1)
|
Present (5; 38.5)
|
Absent
|
Absent
|
0.616
|
|
Massive perivillous fibrin deposition
|
Present (9; 28.1)
|
Present (2; 15.4)
|
Present (1; 100)
|
Present (1; 50.0)
|
0.246
|
|
Maternal floor infarct
|
Present (2; 6.3)
|
Absent
|
Absent
|
Absent
|
0.791
|
|
Retroplacental hematoma
|
Present (1; 3.1)
|
Present (1; 7.7)
|
Absent
|
Absent
|
0.891
|
|
Acute chorionitis of chorionic plate
|
Present (3; 9.4)
|
Present (1; 7.7)
|
Absent
|
Absent
|
0.955
|
|
Acute chorionic plate vasculitis
|
Present (2; 6.3)
|
Present (2; 15.4)
|
Absent
|
Present (1; 50.0)
|
0.220
|
|
Chorionic plate hematoma
|
Absent
|
Absent
|
Absent
|
Absent
|
-
|
Note: there was no statistically significant difference between the stages, indicating a weak association between the lesions and the disease stages. However, the in-stage association was most significant for stage 4 disease subjects, using percentage proportionality.
Discussion
Our study found that the mean, median, and modal ages were near each other. Studies reviewed for this survey found similar age statistics.4,11,12,53 This makes our study comparable.
The test group had their infants mainly through cesarean section (52.1%), while the control group had more spontaneous vaginal deliveries (66.7%), in agreement with previous findings.11,12,53
In both groups the babies, birth weights were within the normal range (2.5–4.5 kg); however, none of their babies were macrosomic (with birth weight > 4.5 kg). The reason for this is unknown.
Importantly, 26.5% of test group babies had LBW (< 2.5 kg) while 9.4% of the control group had LBW infants. This was strongly associated with HIV infection (p = 0.004). This may reflect a compromised in utero placental function from HIV infection. This agrees with various studies within Nigeria and abroad.3,19–27
The test group’s placental weights were mainly within the lowest placental weight bracket of 250–399 g (12.5%) while none of the control group’s placentas were within this weight category. This finding was strongly associated with HIV infection (p = 0.003). This strong association may reflect a compromised in utero perfusion state from HIV infection. Notably, the test and control groups’ mean placental weights (575.5±190.1 g and 664.9±167.4 g) were higher than those reported in similar studies.5,11,12,53 The reason for this finding is unknown and requires further investigation.
The test and control groups’ placental/birth weight ratios were similarly reduced (6.1% and 6.5%, respectively). This finding was weakly associated with HIV infection (p = 0.33), which is in contrast with other studies.14,54 The reason for this unknown and requires further research.
In the test group, fetal membranes showed discoloration and acute inflammatory lesions. Notably, 60.4% of the test group’s fetal membrane had greenish yellow to brown discoloration while 45.3% of the control group’s fetal membranes were discolored. This discoloration indicates meconium staining of the fetal membrane most likely due to fetal distress in utero. Fetal distress may be caused by in utero inflammation from HIV infection. This further illuminates the test group’s higher rate of cesarean section. This finding was weakly associated with HIV infection (p = 0.08), and in contrast with previous studies.11,53 This may reflect better obstetrics/perinatal care.
The test group showed more acute fetal membrane inflammatory lesions than the control group, namely acute chorioamnionitis (47.9% vs. 21.1%) and acute choriodeciduitis (41.7% vs. 8.4%). These findings were strongly associated with HIV infection (p = 0.001 and p < 0.001, respectively). These were consistent with previous studies.2,13 The major etiology of acute inflammatory lesions (particularly suppurative forms) of the fetal membrane is ascending bacterial infection, resulting in maternal inflammatory response.2,13,50 HIV infection lowers the immunity of its host to create a conducive environment for opportunistic infections; hence the strong association of acute fetal membrane inflammatory lesions with HIV infection in this study. HIV-positive mothers will benefit from ascending bacterial infection evaluation. Further research to isolate the possible etiologic agents is needed.
Notably, acute chorioamnionitis was the commonest lesion in the test group the fetal membranes. This was consistent with similar studies.4,11,12,14,18,54,55 However, it was inconsistent with studies that found fetoplacental vasculopathy, marginal infarct, and villitis of unknown etiology as their test group’s commonest placental lesions.5,53 This may reflect lower proportions of opportunistic infections in the test groups because of better preventive/prophylactic and interventional modalities.
Furthermore, acute chorioamnionitis of higher stage and grade occurred more in the fetal membranes of the test group than the control group. Acute suppurative necrotizing chorioamnionitis (stage 3, grade 2) was strongly associated with HIV infection (p < 0.001). Thus, HIV infection engendered a suitable in utero environment for virulent opportunistic microbial agents. In support of this, none of the control group placentas had this stage and grade of chorioamnionitis. This was consistent with previous studies.11,12 Furthermore, the test group placentas (27.1%) showed more PMI than the control group (7.3%). PMI is the combined presence of chorioamnionitis and funisitis and was strongly associated with HIV infection (p = 0.001). This was consistent with the PMI of Schwartz et al.12
Acute chorioamnionitis (47.9%) and acute choriodeciduitis (41.7%) found in the test group suggest that these lesions may occur in one in every two HIV-positive mothers. These lesions may also be of higher stage and grade, having found 20.8% acute suppurative necrotizing chorioamnionitis being stage 3, grade 2.
Importantly, the test group’s umbilical cords displayed more acute inflammatory lesions. These acute inflammatory lesions include acute umbilical phlebitis, acute umbilical panvasculitis, and acute funisitis. This finding tallies with the high proportion of acute inflammatory lesions in the fetal membrane. Notably, whereas acute inflammatory lesions of the fetal membrane signify maternal inflammatory response, acute inflammatory lesions of the umbilical cord signify fetal inflammatory response. These findings were loosely associated with HIV infection (p > 0.05). The reason is unknown and the findings were consistent with previous studies.11,12
Furthermore, the test group’s umbilical cords had a higher stage and grade of acute inflammatory lesions than the control group. Notably, acute panvasculitis (stage 2, grade 1) was strongly associated with HIV infection (p = 0.044). Thus, a strong association is revealed with staging and grading. This staging and grading agree with the concept of ascending opportunistic infection and are consistent with the findings of a previous study.4 The immediate clinical implication of this finding is in ascending opportunistic infection evaluation of HIV-positive mothers’ babies.
In the placental disks of the test group, the most common lesion was acute intervillositis (31.3%). Other lesions included acute chorionic plate vasculitis (10.4%), acute chorionitis of the chorionic plate (8.3%), and acute villitis (6.3%). Notably, acute intervillositis, acute chorionitis of the chorionic plate, and acute villitis signify maternal inflammatory response, while acute chorionic plate vasculitis signifies fetal inflammatory response. This variety of inflammatory lesions on the placental disk shows how an HIV-infected environment is ideal for opportunistic infectious pathogens to trigger a wide range of inflammatory reactions. Amongst all these inflammatory lesions, only acute intervillositis was strongly associated with HIV infection (p < 0.001). This is consistent with the concept of ascending opportunistic infection. The reason for the loose association with HIV infection found for the other lesions is unknown. These findings are consistent with the study by López et al.53 The immediate clinical implication is in the obstetrics care given to HIV-positive mothers.
Furthermore, the test group’s placental disk also had villous vasculopathy (33%), which is characterized by concentric vascular endothelial and fibroblastic proliferative thickening secondary to inflammation, leading to its obstruction, infarction (29.2%), and massive perivillous fibrin deposition (27.1%). Interestingly, these three lesions denote maternal circulatory disorders as categorized by United States and Canadian Academy of Pathology.2 This is consistent with the findings of LBW as well as fetal membrane discoloration (indicative of fetal distress). Notably, villous vasculopathy constituted the most common lesion found in the test groups’ placental disk. This may be due to a hostile in utero inflammatory environment. The high intravascular inflammatory cell traffic occurring in inflammatory response usually led to oxidant collateral vascular damage (i.e., vasculopathy). This finding is consistent with previous studies in which the authors found foetoplacental vasculopathy as the commonest lesion in the placenta of HIV-positive mothers.17,53 The immediate clinical implication is in the biophysical evaluation of HIV-positive mothers to monitor their placental vascular activity, knowing that a good placental vascular network/supply directly relates to good fetal nutrient/oxygen supply as well as waste products excretion. Thus, villous vasculopathy directly affects fetal growth and metabolic functions. These maternal circulatory disorders were loosely associated with HIV infection (p > 0.05) consistent with the study by Schwartz et al.12 Furthermore, the reason for the high proportions of massive perivillous fibrin deposition in both groups is not known, hence an opportunity for more research.
Notably, majority of the test group were in stage 1 HIV/AIDS disease. This may reflect the success of the use of highly active anti-retroviral therapy in their management according to the recommendations of prevention of mother to child transmission program.26,31,34 However, on exploring relationships across all the aspects of the placenta with stages of HIV/AIDS, we found that all the severe inflammatory lesions involved the test group subjects having stage 4 disease. This shows a direct relationship between the stage of HIV/AIDS and the occurrence of severe inflammatory lesions or the sequelae thereof, especially for fetal membrane lesions. This reflects the direct/linear relationship between immune competence/inflammatory response and opportunistic infection. This finding, indeed, casts a dark shadow on the well-being of the test group’s fetuses/neonates because of the higher probability of adverse perinatal outcomes. Indeed, previous studies implicated this inflammatory state, especially histologic chorioamnionitis, in the occurrence of vertical transmission of HIV from mother to child in utero through the placenta.16,18,55 Furthermore, studies (particularly Nigerian studies) show that these adverse fetal/perinatal outcomes include spontaneous abortion, stillbirth, intrauterine growth restriction, LBW, small for gestational age, preterm birth, neonatal sepsis, and neonatal encephalopathy.25–27 The immediate clinical implication is needed for closer monitoring of HIV-positive mothers’ clinical states, placental functions, and their fetuses (particularly in stage 4 disease) to ensure good pregnancy outcomes.
This study has shown that HIV infection can cause alterations/lesions (directly or indirectly) in placental anatomy; thus, affirming that altered histomorphometric parameters and lesions in the placenta of HIV-positive mothers and the associated adverse perinatal outcome are present in Uyo, Akwa Ibom State. This has provided the needed data to commence the filling of the identified knowledge gap. This study has also provided data to use in the attainment of goal three of the United Nation’s Sustainable Development Goals.
The limitations of this study were derived basically from the structure of its study design. Being a prospective hospital-based study, the findings cannot be accurately extrapolated to the general population. The maternal obstetrical and perinatal data were extracted from the subjects’ case notes; the difficulties encountered ranged from inadequate documentation to missing case notes. Also, the accuracy of the case notes’ data largely depended on the competence of the clinicians and midwives in documenting it. Additionally, though yellow-brown fetal membrane discoloration was found, we were not able to histopathologically evaluate for meconium. Furthermore, autopsies were not performed on the macerated and fresh stillbirths to evaluate effects of HIV.
Conclusion
One-quarter (26.5%) of babies in the test group were in the LBW category, and 12.5% of their placentas weighed > 400 g. Their placental fetal membrane, umbilical cord, and disk showed acute chorioamnionitis, acute panvasculitis (stage 2, grade 1), and acute intervillositis as strongly associated lesions with HIV infection. Though there is a linear relationship between the severity of placental histopathological lesions and stage of HIV/AIDS, no significant association was found.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
This original article was gleaned from the principal investigator’s National Postgraduate Medical College of Nigeria part 2 final fellowship examination (FMCPath) in Pathology (Anatomical Pathology) dissertation research work on histopathological changes in placentas of HIV-positive mothers; for which he is immensely grateful to his research assistant/medical laboratory scientists (Oyedele Oyewumi Ajayi and Fidelis Onyem Ngari), and the management staff of the department of Histopathology, UUTH, Uyo, Akwa Ibom
State, Nigeria.
references
- 1. Gupta D, Bhatnagar S, Mishra S. Euthanasia: issues implied within. Internet J Pain Symptom Control Palliat Care 2006;4(2).
- 2. Huettner PC. 2008 Short Course #06 - placental development, indications for and methods of examination. United States and Canadian Academy of Pathology. 2008 [cited 2015 Oct 23]. p. 1-80. Available from: http://www.uscapknowledgehub.org/newindex.htm?97th/shorth.htm.
- 3. Ackerman W, Kwiek JJ. Role of the placenta in adverse perinatal outcomes among HIV-1 seropositive women. J Nippon Med Sch 2013;80(2):90-94.
- 4. Anderson VM, Zevallos E, Gu J. The HIV-exposed placenta morphologic observations and interpretation. Placenta 1994;15:47-65.
- 5. Vermaak A, Theron GB, Schubert PT, Kidd M, Rabie U, Adjiba BM, et al. Morphologic changes in the placentas of HIV-positive women and their association with degree of immune suppression. Int J Gynaecol Obstet 2012 Dec;119(3):239-243.
- 6. Baurakiades E, Martins AP, Victor Moreschi N, Souza CD, Abujamra K, Saito AO, et al. Histomorphometric and immunohistochemical analysis of infectious agents, T-cell subpopulations and inflammatory adhesion molecules in placentas from HIV-seropositive pregnant women. Diagn Pathol 2011 Oct;6(1):101.
- 7. AETCNCRC. HIV Classification: CDC and WHO staging systems. AIDS Education and Training Centers, National Coordinating Resource Center (AETC NCRC). 2014 [cited 2015 Nov 4]. p. 1. Available from: http://aidsetc.org/guide/hiv-classification-cdc-and-who-staging-systems.
- 8. Al Hasani NA, Al Dughaishi T, Balkhair AA. HIV and pregnancy: a retrospective descriptive cross-sectional study of prevalence, maternal, obstetrical, and neonatal outcome at a tertiary care hospital in Oman. Oman Med J 2021 Nov;36(6):e321.
- 9. WHO. WHO case definition of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization; 2007.
- 10. WHO. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance, African region. World Health Organization. Geneva: World Health Organization; 2005. p. 1-49.
- 11. Schuetz AN, Guarner J, Packard MM, Zaki SR, Shehata BM, Opreas-Ilies G. Infectious disease immunohistochemistry in placentas from HIV-positive and HIV-negative patients. Pediatr Dev Pathol 2011 May-Jun;14(3):180-188.
- 12. Schwartz DA, Sungkarat S, Shaffer N, Laosakkitiboran J, Supapol W, Charoenpanich P, et al. Placental abnormalities associated with human immunodeficiency virus type 1 infection and perinatal transmission in Bangkok, Thailand. J Infect Dis 2000 Dec;182(6):1652-1657.
- 13. Salafia CM, Popek EJ. Inflammatory and vascular placental pathology. Glob Libr Women’s Med. 2008 [cited 2015 Oct 26]. Available from: http://www.glowm.com/section_view/heading/Inflammatory%2520and%2520Vascular%2520Placental%2520Pathology/item/152.
- 14. D’costa GF, Khadke K, Patil YV. Pathology of placenta in HIV infection. Indian J Pathol Microbiol 2007 Jul;50(3):515-519.
- 15. Nagi AH. Monograph: placenta examination and pathology. Biomedica 2011;27:81-99.
- 16. Bhoopat L, Khunamornpong S, Sirivatanapa P, Rithaporn T, Lerdsrimongkol P, Thorner PS, et al. Chorioamnionitis is associated with placental transmission of human immunodeficiency virus-1 subtype E in the early gestational period. Mod Pathol 2005 Oct;18(10):1357-1364.
- 17. Bittencourt AL, Garcia AG. The placenta in hematogenous infections. Pediatr Pathol Mol Med 2002 Jul-Aug;21(4):401-432.
- 18. Ferrero S, Fulcheri E, Bentivoglio G, Parodi A. Pathologic lesions of the placenta and HIV vertical transmission. In: infectious diseases society of America 2003 Annual Meeting. 2003 [cited 2015 Oct 30]. Available from: http://www.researchgate.net/publication/267519104_Pathologic_Lesions_of_the_Placenta_and_HIV_Vertical_Transmission.
- 19. Aaron E, Bonacquisti A, Mathew L, Alleyne G, Bamford LP, Culhane JF. Small-for-gestational-age births in pregnant women with HIV, due to severity of HIV disease, not antiretroviral therapy. Infect Dis Obstet Gynecol 2012;2012(12):135030.
- 20. Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, et al. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania. Am J Clin Nutr 2010 Feb;91(2):391-397.
- 21. Kennedy D, Fawcus S, Kroon M. The effect of maternal HIV status on perinatal outcome at Mowbray Maternity Hospital and referring midwife obstetric units, Cape Town. S Afr J Obstet Gynaecol. 2012 [cited 2015 Oct 25]. Available from: http://www.highbeam.com/doc/1G1-281375507.html.
- 22. Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. BJOG An Int J Obstet Gynaecol1998;105(8):836-48.
- 23. Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis 2005 Dec;41(11):1654-1661.
- 24. Aiken CG. HIV-1 infection and perinatal mortality in Zimbabwe. Arch Dis Child 1992 May;67(5):595-599.
- 25. Olagbuji BN, Ezeanochie MC, Ande AB, Oboro VO. Obstetric and perinatal outcome in HIV positive women receiving HAART in urban Nigeria. Arch Gynecol Obstet 2010 Jun;281(6):991-994.
- 26. Joseph O, Biodun O, Michael E. Pregnancy outcome among HIV positive women receiving antenatal HAART versus untreated maternal HIV infection. J Coll Physicians Surg Pak 2011 Jun;21(6):356-359.
- 27. Uneke CJ, Duhlinska DD, Ujam TN. Effects of maternal plasmodium falciparum malaria and HIV infection on birth weight in Southeastern Nigeria. Vol. 12, McGill Journal of Medicine (MJM) An International Forum for the Advancement of Medical Sciences by Students. 2009 [cited 2015 Oct 25]. p. 42. Available from: http://europepmc.org/articles/PMC2997242.
- 28. UNFPA. Sexual and Reproductive Health. UNFPA Nigeria. 2015 [cited 2015 Oct 19]. p. 1. Available from: http://nigeria.unfpa.org/index.php?url=scope&p=srh.
- 29. UNAIDS. Fact sheet: global HIV/AIDS statistics. UNAIDS. 2015 [cited 2015 Nov 7]. Available from: http://www.unaids.org/en/resources/campaigns/2014/2014gapreport/factsheet.
- 30. AkwaIbomSACA. HIV/AIDS information. Akwa Ibom State agency for the control of AIDS. 2013 [cited 2013 Jun 23]. p. 1. Available from: http://akwaibomsaca.com/info.htm.
- 31. Markson JA, Umoh AV. Evaluation of PMTCT programme implementation in General Hospital, Iquita, Oron, Akwa Ibom State, Nigeria. Ibom Med J. 2013;6(1):5-15.
- 32. Abasiattai AM, Umoiyoho AJ, Udoma EJ, Abasiubong FS, Ukafia S. Prevalence of HIV infection among antenatal attendees at Uyo Teaching Hospital, Akwa Ibom State, South-South Nigeria. Glob J Community Med 2009;2(1–2):47-51.
- 33. Etok C, Etukafia M, Mboto C. Sero – prevalence of human immunodeficency virus (HIV) infection among antenatal clinic attendees in St Luke’s Hospital, Uyo, Akwa Ibom State, Nigeria. Glob J Pure Appl Sci 2010;16(2).
- 34. Ogungbemi MK, Sagbohan J, Adeyemi A, Agbo F, Ogbe A, Okosun L, et al. United Nations General Assembly Special Session (UNGASS) Country Progress Report: Nigeria. Nigeria; 2010 [cited 2015 Oct 26]. Available from: http://data.unaids.org/pub/Report/2010/nigeria_2010_country_progress_report_en.pdf.
- 35. Awofala AA, Ogundele OE. HIV epidemiology in Nigeria. Saudi J Biol Sci 2018 May;25(4):697-703.
- 36. Negedu-Momoh OR, Balogun O, Dafa I, Etuk A, Oladele EA, Adedokun O, et al. Estimating HIV incidence in the Akwa Ibom AIDS indicator survey (AKAIS), Nigeria using the limiting antigen avidity recency assay. J Int AIDS Soc 2021 Feb;24(2):e25669.
- 37. UNDP. Sustainable Development Goals (SDGs). UNDP. 2015 [cited 2015 Nov 1]. Available from: http://www.undp.org/content/undp/en/home/mdgoverview/post-2015-development-agenda.html.
- 38. UN. SDGs and Topics: sustainable development knowledge platform. UN Sustainable Development Knowledge Platform. 2015 [cited 2015 Nov 1]. Available from: https://sustainabledevelopment.un.org/topics.
- 39. Rosai J. Guidelines for handling of most common and important surgical specimen. In: Rosai J, editor. Rosai and Ackerman’s Surgical Pathology. 9e. Vol. 2. India: Mosby, An Imprint of Elsevier; 2004. p. 2957-2958.
- 40. Gersell DJ, Kraus FT. Diseases of the placenta; examination of the placenta. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6th ed. New York: Springer Science & Business Media; 2011. p. 1067-1069.
- 41. Dahlstrom J, Charlton A, Arbuckle S. Macroscopic cut-up manual: placenta multiple. The Royal College of Pathologist of Autralasia (RCPA). 2016 [cited 2015 Dec 4]. Available from: http://www.rcpa.edu.au/Library/Practising-Pathology/Macroscopic-Cut-Up/Specimen/Gynaecology-and-perinatal/Placenta/Placenta-multiple.
- 42. Lester SC. Gynaecologic and perinatal pathology. In: Lester SC, editor. Manual of surgical pathology. USA: Churchill Livingstone: a Harcourt Health Sciences Company; 2001. p. 256-259.
- 43. Benirschke K, Kaufmann P, Baergen R. Pathology of the human placenta. 5th ed. Benirschke K, Kaufmann P, Baergen R, editors. New York, NY: Springer Science+Business Media, Inc; 2006. p.1027.
- 44. Roberts DJ. Placental pathology, a survival guide. Arch Pathol Lab Med 2008 Apr;132(4):641-651.
- 45. Ziadie M. Placenta - Grossing placentas. Pathology Outlines. 2011 [cited 2015 Oct 26]. p. 1. Available from: http://www.pathologyoutlines.com/topic/placentagrossing.html.
- 46. Roberts DJ. Introduction. In: Lockwood CJ, Garcia RL, Barss VA, editors. UpToDate: gross examination of the placenta. Wolters Kluwer Health Clinical Solutions; 2015 [cited 2015 Oct 26]. p. 1. Available from: http://www.uptodate.com/contents/gross-examination-of-the-placenta.
- 47. Heazell A. Abnormalities of the placenta. BMC pregnancy childbirth 2012;12(Suppl 1):A2. http://www.biomedcentral.com/1471-2393/12/S1/A2.
- 48. Redline RW, Heller D, Keating S, Kingdom J, Redline RW, Faye-Petersen O, et al. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta 2005;26 Suppl A:S114-S117.
- 49. Redline RW, Matzinger P, Gruenwald P, Pijnenborg R, Caniggia I, Winter J, et al. Placental pathology: a systematic approach with clinical correlations. Placenta 2008 Mar;29(Suppl A):S86-S91.
- 50. Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C; Society for pediatric pathology, perinatal section, amniotic fluid infection nosology committee. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003 Sep-Oct;6(5):435-448.
- 51. Panti AA, Ekele BA, Nwobodo EI, Yakubu A. The relationship between the weight of the placenta and birth weight of the neonate in a Nigerian Hospital. Niger Med J 2012 Apr;53(2):80-84.
- 52. Perry IJ, Beevers DG, Whincup PH, Bareford D. Predictors of ratio of placental weight to fetal weight in multiethnic community. BMJ 1995 Feb;310(6977):436-439.
- 53. López CL, Pires AR, de Fonseca EC, Rodrigues FR, Braga Neto AR, Herdy GV, et al. Anatomopathological characterization of placentas from HIV+ patients associated with p24 expression. J Bras Patol Med Lab 2013;49(6):437-445. http://www.scielo.br/scielo.php?script=sci_ar
- 54. Jauniaux E, Nessmann C, Imbert MC, Meuris S, Puissant F, Hustin J. Morphological aspects of the placenta in HIV pregnancies. Placenta 1988 Nov-Dec;9(6):633-642.
- 55. Mwanyumba F, Gaillard P, Inion I, Verhofstede C, Claeys P, Chohan V, et al. Placental inflammation and perinatal transmission of HIV-1. J Acquir Immune Defic Syndr 2002 Mar;29(3):262-269.