Gallbladder disease is relatively common in women, and pregnancy has been shown to contribute to forming gallstones in females.1–4 The presence of sludge and gallstones predispose to cholecystitis, gallstone pancreatitis, and other morbidities and may require surgical interventions, increase patient stay in the hospital, and result in frequent hospital visits during pregnancy and trigger preterm deliveries. In rare cases, maternal mortality, premature delivery, and fetal loss may also occur.5–7
Hormonal changes such as increased serum levels of progesterone occur during pregnancy, especially during the third trimester. These changes cause impaired gallbladder relaxation; the resultant effect is increased gallbladder volume and stasis of bile which predispose to gallstone formation.8–11 Other factors that may result in alteration of gallbladder functions during pregnancy are high estrogen levels and impaired contractility due to cholecystokinin secretion.12,13
Previous studies have shown that maternal age, parity, body mass index (BMI), and oral contraceptive use were implicated in forming gallstones in pregnancy.14–16 This study was conducted using ultrasonography to determine the influence of these risk factors on gallbladder volume in pregnancy in Ilorin, Nigeria. Ultrasonography (non-ionizing and non-invasive) helps in the early detection of large gallbladder volume and poor contractility and also helps identify high-risk patients.8,17–19 This is relevant for early commencement of preventive measures, such as dietary modification, weight reduction, and physical activity, thereby preventing gallstone formation.20 Furthermore, for patients who may develop gallbladder disease in pregnancy, prompt management by obstetricians can prevent complications of gallbladder disease to the fetus and mother. Management options may include discontinuation of oral intake of fluids and solids, intravenous fluid replacement, analgesia, antibiotics treatment, or surgical interventions (cholecystectomy).20–22
Methods
This study was a prospective cross-sectional study in design. It was carried out in the Fetal Assessment Unit of the Department of Radiology, University of Ilorin Teaching Hospital, Nigeria from May 2016 to October 2016. A total of 190 pregnant women between 32 and 40 weeks gestation referred for a routine obstetric scan from the Department of Obstetrics and Gynecology were recruited. Gestational age was evaluated using the last menstrual period and/or early trimester ultrasound scan.
The study procedure and benefits were thoroughly explained to patients during antenatal visits. They were told to observe at least 12 hours overnight fast before the day of the study (to ensure satisfactory gallbladder distension and to reduce the amount of gastric and intestinal gas) and that two upper abdominal ultrasound scans will be carried out before and after ingesting two sachets of Three Crowns Filled evaporated milk. Consenting patients were recruited consecutively until the desired sample size was obtained.
The sample size was determined using Leslie Fisher’s formula as follows:
Where z = standard normal deviation set at 1.96, which corresponds to a 95% CI.
p = prevalence of gallstone in pregnancy in a city in Nigeria (2.9%)2
q = 1.0-p
d = the degree of accuracy required is usually set at 0.05
n = [1.96 × 1.96 × 0.029 × 0.971]/[0.05 × 0.05] = 43
The sample size was increased to 190 to improve the validity of the study. Patients with comorbid conditions such as diabetes mellitus, sickle cell anemia, hypertension, and pre-eclampsia, including patients with other systemic illnesses such as gastrointestinal, gallbladder, liver, or other endocrine diseases, were excluded from the study. Patients taking medications known to affect gallbladder emptying such as calcium channel blockers, opioids, anticholinergics, progestogens and estrogens, post-cholecystectomy patients, patients with allergies to liquid evaporated milk, and patients with multiple pregnancies were also excluded from the study.
A self-designed form was used to record biodata, obstetric history, medical history, fasting and postprandial gallbladder volumes (FGBV and PGBV), and ejection fraction (EF). In addition, all booking investigations such as hemoglobin genotype (to rule out sickle cell anemia), urinalysis (to rule out pre-eclampsia), and blood sugar estimation (for patients at risk such as patients family history of diabetics and obese patients) were reviewed.
Weight and height were measured using a standard weighing scale and stadiometer. BMI was calculated as weight (kg)/height (m).2
We used a Siemens Sonoline SI-400 ultrasound scanner with a 3.5 MHz curvilinear probe and acoustic coupling gel for ultrasound studies. Patients were positioned supine on an examination couch, and the abdomen was exposed to the pubic line. After applying coupling gel to the skin, a brief obstetric scan was done to ascertain the gestational age. This was determined using a combination of multiple biometric parameters (biparietal diameter, femur length, and abdominal circumference), and compared with published western nomogram (Hadlock’s) for each parameter.23
Compliance with fasting was ascertained by scanning the stomach and duodenal regions for food residue or fluid. Two serial right upper quadrant scans were subsequently done to assess the maternal gallbladder: before drinking 120 mL (two sachets) of Three Crowns Filled Evaporated Milk (produced by FrieslandCampina WAMCO Nigeria PLC®) containing 10.2 g of fat, 8.9 g of protein, and 14.3 g of carbohydrate and 30 minutes postprandial.

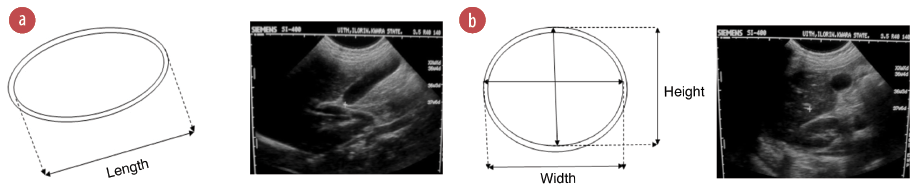 Figure 1: (a) Line diagram and ultrasound (US) image of a longitudinal view of the gallbladder showing length measurement. (b) Line diagram and US image of the transverse view of gallbladder showing width and height measurements.
Figure 1: (a) Line diagram and ultrasound (US) image of a longitudinal view of the gallbladder showing length measurement. (b) Line diagram and US image of the transverse view of gallbladder showing width and height measurements.
The length of the gallbladder was measured on breath holding using the maximum longitudinal dimension, either in supine or right anterior oblique position [Figure 1a]. The probe was rotated through 90º to obtain the maximal transverse dimensions (i.e., the width and height, with the calipers crossing each other at 90º) [Figure 1b]. Inner to outer wall dimensions were used for all measurements. Measurements were taken thrice and the average for each was recorded. Measurements of gallbladder dimensions were repeated 30 minutes postprandial.
Gallbladder volumes (fasting and postprandial) were obtained using the ellipsoid method (volume = length × width × height × 0.523).24 Ejection at 30 minutes was calculated by the following equation:
EF8 = (FGBV-PGBV)/FGBV × 100. Ethical approval to conduct the study was obtained from the Ethical Review Committee of the University of Ilorin Teaching Hospital, Ilorin, Nigeria.
The data was entered and analyzed using the SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Gallbladder volume and EF were the dependent variables with parity, maternal age, BMI, and previous oral contraceptive use as independent variables. The strength and the direction of the relationship between the gallbladder volume and EF; parity and BMI were analyzed using Pearson’s correlation coefficient. Spearman’s correlation coefficient was used for its relationship with age, while its relationship with previous oral contraceptive use was analyzed using the independent samples t-test. P-values < 0.05 were considered statistically significant.
Results
A total number of 190 participants were evaluated. The age of the participants ranged from 16–45 years, with a mean age of 29.53±6.15 years [Table 1]. Most participants (31.0%) were primiparous (parity 1). The mean gestational age was 35.45±2.28 weeks. Fewer respondents (31.8%) had histories of previous oral contraceptive pill (OCP) use. A mean BMI of 26.87±5.48 was obtained. The mean FGBV was 46.76±21.28 mL, while PGBV was 21.14±13.26. A mean EF of 49.81±65.76 was obtained.
Table 1: Age of study participants.
|
Age group, years
|
|
|
|
16–20
|
8
|
4.2
|
|
21–25
|
38
|
20.0
|
|
26–30
|
75
|
39.5
|
|
31–35
|
36
|
18.9
|
|
36–40
|
22
|
11.6
|
Mean age of participant: 29.53±6.15 years.
Maternal age showed weak positive correlations with FGBV (r = 0.077) and PGBV (r = 0.068). A weak negative correlation was noted with EF (r = -0.029). However, there was no statistically significant relationship between maternal age and gallbladder parameters; FGBV, PGBV, and EF (p = 0.305, 0.369, and 0.699, respectively), as shown in Table 2.
Parity showed weak positive correlations with FGBV (r = 0.102), PGBV (r = 0.034), and EF (r = 0.014) that were not statistically significant (p = 0.166, 0.649, and 0.852, respectively) [Table 2]. BMI showed statistically significant positive, though weak correlations with FGBV (r = 0.179, p = 0.015) and PGBV (r = 0.216, p = 0.003). A positive correlation (r = 0.520) was also observed with EF but was not statistically significant (p = 0.478) [Table 2]. Previous oral contraceptive use showed a weak positive association with FGBV (t = 0.067), but a strong positive association was observed with PGBV (t = 0.981) and EF (t = 0.655). However, there was no statistically significant relationship between previous OCP use and the gallbladder parameters; FGBV, PGBV, and EF (p = 0.967, 0.328, and 0.513, respectively) [Table 3].
Prompt identification of possible sociodemographic and anthropometric factors that may influence gallbladder volumes in pregnancy is vital to prevent gallbladder disease in pregnancy and for the early management of patients who may develop gallbladder disease. There was no statistically significant correlation between maternal age and gallbladder volumes. This was contrary to a study in Italy on healthy adults, where gallbladder volume was shown to correlate positively with age.25 This was also at variance with a related study in Nairobi, Kenya.26
A similar study in people with diabetes observed that the positive correlation between age and gallbladder volume resulted from spontaneous autonomic denervation of the gallbladder with aging, leading to hypomotility, bile stasis, and increased gallbladder volume.27 However, differences in subject population may be the reason for the contrary findings in this study. Furthermore, there was also no significant correlation between maternal age and gallbladder EF, similar to previous findings.28 This finding also agrees with those of Wedman et al.29
Higher parity is associated with increased lifetime exposure to estrogen, which alters the composition of bile and impairs biliary motility (or EF) with resultant increased gallbladder volume and gallstone formation.30 However, this study does not show any statistically significant correlation between parity, gallbladder volumes, and EF. This agrees with a study on gallbladder dysmotility in pregnancy.31 A similar study in pregnant women could only demonstrate a significant correlation between parity and gallbladder EF.20 Agarwa et al,27 showed a significant correlation between parity and gallbladder volume. Nonetheless, their study was done on diabetic patients.27 Most of the study participants were primiparous, which may be the reason for the contrary findings in this study.
BMI showed a statistically significant but weakly positive correlation with gallbladder volumes in this study. A strong relationship between gallbladder volume and BMI was reported in studies on gallbladder volume in obese women in Turkey.32 Similar findings were observed in another study on healthy adults in Kano, Nigeria.33 Then again, Adeyeku and Ukadike in Benin, Nigeria, did not show any correlation between BMI and gallbladder volume.34 Other researchers have demonstrated greater gallbladder volumes with increased BMI. It has been documented that higher BMI is associated with excessive body visceral fat and insulin resistance, which may predispose to gallbladder motility defects. Hence, large gallbladder volumes, gallbladder stasis, and gallstone formation.35 In addition, autonomic neuropathy or reduced gallbladder sensitivity to cholecystokinin may also be contributory.36 The relatively weak positive but statistically significant correlation between gallbladder volume and BMI in this study may be due to dietary factors or racial differences. Nevertheless, no statistically significant relationship between BMI and EF was obtained in this study. This is in accordance with a previous study by Stone et al,37 but at variance with results obtained in the study by Ugwu et al.28 Marzio et al,38 observed reduced contractility (or EF) in obese patients than controls, and similar results were obtained by Fraquelli et al.36 The contrary findings in this study may be due to difference in study population or racial differences.
Previous studies have shown that estrogen and progesterone, both active components of OCPs, cause decreased gallbladder contractility, which impedes biliary flow, increasing gallbladder volume and resultant gallstone formation.39,40 However, this study showed no significant association between previous oral contraceptive use and gallbladder volume and contractility. This is in accordance with a related study by Braverman et al.41 Pansini et al,42 also in a similar study in healthy non-pregnant women showed no significant difference in gallbladder volume between previous users and non-users of OCP. Few respondents reported a positive history of previous OCP use, and this may be responsible for the non-association between previous OCP use, gallbladder volume, and contractility.
The study limitation is that the response rate regarding the use of OCP was poor, so our results must be interpreted with caution, and a conclusion cannot be drawn.
Among the sociodemographic and anthropometric factors, only BMI showed a statistically significant but weakly positive correlation with gallbladder volume in pregnancy. Thus, the study demonstrated that pregnancy gallbladder volume might depend on BMI. Consequently, high BMI may predispose to large gallbladder volume with a resultant increased risk of biliary stasis and gallstone formation in pregnancy. During obstetric ultrasound examination, the maternal gallbladder should be evaluated quickly, especially in patients with risk factors such as high BMI. This is relevant for the early commencement of preventive measures such as dietary modifications, weight reduction, and physical activity to prevent gallbladder disease during pregnancy. This is also necessary for follow-up and expectant management of patients that may develop gallbladder disease during pregnancy. Management options include conservative (discontinuation of oral intake, intravenous fluid replacement, analgesia, and antibiotics treatment) or surgical (cholecystectomy) approach. However, there is a paucity of data on the influence of sociodemographic and anthropometric factors on gallbladder volume in pregnancy. There is, therefore, the need for further multicentre research to obtain data to compare with the results of this study and generate more generally acceptable reference data.
The authors declared no conflicts of interest. No funding was received for this study.