The oral cavity environment is full of normal flora and some opportunistic bacteria. Microbial colonization on the teeth is a major source of pathogens responsible for periodontal disease.1 Periodontitis is a serious gum inflammation that damages the soft tissue in response to bacterial infections. It is a chronic disease that affects a major part of the world population. Periodontal disease is also associated with other conditions like heart diseases and other systemic diseases.2,3
The main target of periodontal disease treatment is to eliminate the pathogens responsible for infection and decrease tissue inflammation. Antimicrobial agents and non-steroidal anti-inflammatory drugs have been used to treat periodontal disease.4–7
Local delivery of moxifloxacin against bacterial periodontitis was successfully developed as an effective site-specific drug delivery system for periodontitis treatment.8
Metronidazole gel 25% showed a major decrease in the probing depth when compared with the control groups’ local therapies.9
Thermoreversible hydrogel loaded with anti-inflammatory lipoxin A4 and/or antibiotic doxycycline reduced bacterial load and pro-inflammatory interleukin-8 level. This finding showed that the thermoreversible hydrogel is a safe and effective vehicle for periodontal drug delivery.10
An experiment on periodontal disease should be established on animal gum to study the disease response. One of the methods to induce periodontitis is the ligature model.11 Induction was achieved through fixing ligature using silk or cotton around the teeth on the submarginal position of the necks of the teeth. The advantage of the ligature model is that disease can be initiated and facilitated at a known time with a predictable sequence of events of inflammation of the periodontal ligaments within a few days.12 The removal of the ligature helped investigate the resolution of inflammation and the healing response after applying the treatment.13–15
We sought to evaluate the healing efficacy of syringable, in situ gelling formulations containing metronidazole, diclofenac potassium, or a combination of the two drugs on ligature-induced periodontitis (LIP) in rats. Before and after treatment, the inflammation parameters, namely, presence of polymorphonuclear (PMN) cells, ligament organization, granulation, necrosis, and edema were assessed [Table 1].
Table 1: Results of scoring system for periodontal tissue-healing parameters in control, untreated, and treated rats.
|
Control group |
1 |
0 |
Organized |
0 |
0 |
0 |
0 |
|
2 |
0 |
Organized |
0 |
0 |
0 |
0 |
|
3 |
0 |
Organized |
0 |
0 |
0 |
0 |
|
4 |
0 |
Organized |
0 |
0 |
0 |
0 |
|
5 |
0 |
Organized |
0 |
0 |
0 |
0 |
|
6 |
0 |
Organized |
0 |
0 |
0 |
0 |
|
Mean |
0.0 |
|
0.0 |
0.0 |
0.0 |
0.0 |
|
SD |
0.0 |
|
0.0 |
0.0 |
0.0 |
0.0 |
|
Untreated Rat |
1 |
3.00 |
Disorganized |
2.00 |
0.00 |
2.00 |
2.00 |
|
2 |
3.00 |
Disorganized |
2.00 |
0.00 |
3.00 |
1.00 |
|
3 |
3.00 |
Disorganized |
1.00 |
0.00 |
2.00 |
2.00 |
|
4 |
3.00 |
Disorganized |
2.00 |
0.00 |
3.00 |
2.00 |
|
Mean |
3.0 |
|
1.7 |
0.0 |
2.5 |
1.7 |
|
SD |
0.0 |
|
0.5 |
0.0 |
0.5 |
0.5 |
|
Metronidazole loaded gel formulation |
1 |
2.00 |
Disorganized |
1.00 |
1.00 |
1.00 |
0.00 |
|
2 |
1.00 |
Disorganized |
0.00 |
2.00 |
1.00 |
0.00 |
|
3 |
2.00 |
Disorganized |
1.00 |
2.00 |
1.00 |
0.00 |
|
4 |
1.00 |
Disorganized |
1.00 |
2.00 |
1.00 |
0.00 |
|
Mean |
1.5 |
|
0.7 |
1.7 |
1.0 |
0.0 |
|
SD |
0.5 |
|
0.5 |
0.5 |
0.0 |
0.0 |
|
Diclofenac potassium loaded gel formulation |
1 |
1.00 |
Disorganized |
1.00 |
0.00 |
1.00 |
0.00 |
|
2 |
2.00 |
Disorganized |
1.00 |
1.00 |
0.00 |
0.00 |
|
3 |
2.00 |
Disorganized |
1.00 |
0.00 |
0.00 |
0.00 |
|
4 |
1.00 |
Disorganized |
1.00 |
1.00 |
1.00 |
0.00 |
|
Mean |
1.5 |
|
1.0 |
0.5 |
0.5 |
0.0 |
|
SD |
0.5 |
|
0.0 |
0.5 |
0.5 |
0.0 |
|
1 |
1.00 |
Organized |
1.00 |
3.00 |
0.00 |
0.00 |
|
2 |
0.00 |
Organized |
1.00 |
3.00 |
1.00 |
0.00 |
|
3 |
0.00 |
Organized |
0.00 |
3.00 |
0.00 |
0.00 |
|
4 |
1.00 |
Organized |
1.00 |
3.00 |
1.00 |
0.00 |
PMN: polymorphonuclear.
Methods
Poloxamer 188 and poloxamer 407 were obtained from BASF Chemicals Company, Limburgerhof, Germany. Metronidazole was purchased from Ranbaxy, India. Diclofenac potassium and xanthan gum were obtained from National Pharmaceutical Industries, Muscat, Oman. HR40 non-resorbable sterile silk thread (polyamide monofilament nonabsorbable or Barbour’s linen suture thread) was obtained from Huaian Conley Properties, Wanjia, China.
A thermosensitive in situ gelling formulation containing 30% poloxamer (10% poloxamer 188 and 20% poloxamer 407) and 2% w/w xanthan gum was prepared using the cold method.7
Sets of in situ gelling formulations containing 30% poloxamer 188 and 407 were prepared, and xanthan gum was incorporated at concentrations of 1.5%, 1.8%, and 2%. It was found based on comparative in vitro release studies that the 2% formulation was the optimized gel formulation, and this was used for further studies. Metronidazole (5% w/w), diclofenac potassium (0.2% w/w), or a combination of metronidazole (5% w/w) and diclofenac potassium (0.2%) were incorporated to prepare three thermosensitive in situ gelling formulations. The formulations were stored in polyethylene containers at 8 °C until used.
A volume of 3 mL of the gelling formulations was measured into a 15 mL centrifuge tube and heated in a water bath. The temperature at which the gel did not fall when the tube was turned 90º was recorded as the gelation temperature. The experiment was carried out in triplicate.7
The work required to expel the formulation from a syringe (without needle) was determined in compression mode using Texture analyzer (TA-TX Plus, Stable Micro System, UK). The sample was packed into a 3 mL polyvinyl chloride syringe (Terumo, Indonesia) to a height of 2.5 mL. The syringe was then placed in metallic support and immobilized with a metal clamping ring. The plunger of a syringe was attached to the arm of the Texture analyzer by probe adaptor at the height of 40 mm from the syringe. The plunger descended at a constant speed of 5 mm/s and expelled the gel from the syringe before returning to its original position [Figure 1].13 The work of syringeability was calculated from the area under the resultant force-time plot.16–18 The experiment was performed in triplicate.
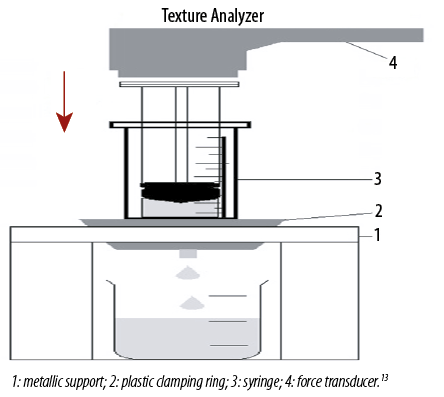 Figure 1: Experimental set-up for measurement of syringeability.
Figure 1: Experimental set-up for measurement of syringeability.
The in vitro release of metronidazole and diclofenac potassium was determined using a dissolution tester apparatus I basket method (Pharma Test, PTWS 3CE, Hainburg, Germany). One mL of gel formulation was measured into a muslin cloth and tied with thread before placing inside the basket. Glass tubes of 6.0 cm diameter and 17.0 cm height were used as dissolution vessels. The basket was connected to the dissolution tester and submerged in the glass tube. The study was carried out at 37.0±0.5 °C with a rotation speed of 100 rpm in 100 mL of 0.05M potassium phosphate buffer (pH 6.8) dissolution medium. Sample of 5 mL was collected from the dissolution vessel at preset time intervals of 1, 2, 3, 4, 5, 6, 24, 48, 72, 96, 120, 144, and 168 hours and immediately refilled with the same volume of fresh dissolution medium. The samples were analyzed using a validated high-performance liquid chromatography method.19 The percentage of drugs released over time was calculated and plotted.
The rats were supplied by the Animal Research and Service Center, USM. They showed healthy, intact teeth and no previous problem with periodontal disease. Five rats were housed in one plastic cage under standard conditions (12 h light/dark cycle and 22±2 °C), with free access to commercial standard rodent chow. Tap water was given ad libitum. They were allowed to adapt to the laboratory environment for about five days before the experiment. The study protocol was approved by the Animal Ethics Committee, School of Pharmaceutical Sciences, USM, Penang, Malaysia (Clearance No (103)(805), 2016).
Thirty adult male Sprague-Dawley rats (220±20 g body weight) were used. A total of 24 rats were subjected to LIP, while the remaining six rats were used as a control group.20 General anesthesia was achieved through intramuscular injection of 0.2 mL/100 g body weight containing 10% ketamine and 2% xylazine (100 mg/kg and 10 mg/kg, respectively) in a ratio of 2:1.21 Ligatures in “8” with HR40 non-resorbable sterile silk thread was placed around the cervix of the lower incisor frontal tooth and knotted on the buccal side.21,22
The clinical manifestation of periodontitis was observed on day five, which included edema and redness of gingiva and bleeding upon probing [Figure 2].

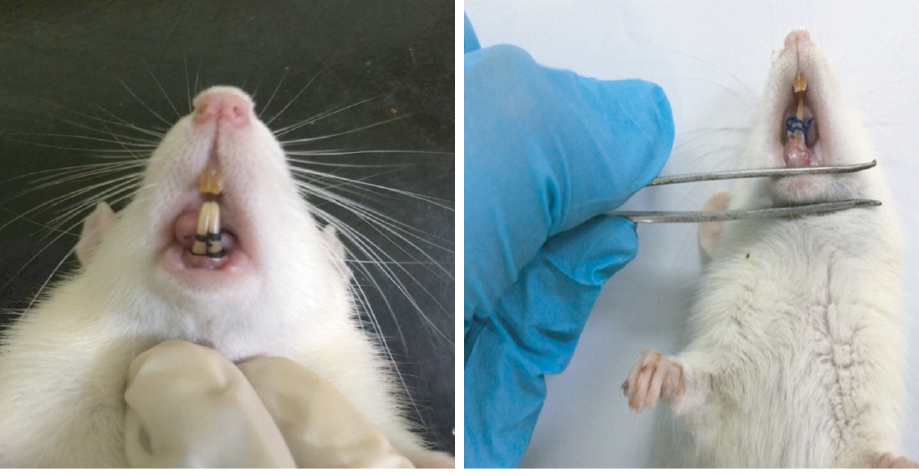 Figure 2: (a) Ligature in the frontal lower incisor tooth of the rats. (b) Examination of inflammation in the frontal lower incisor tooth of a rat five days post-ligature.
Figure 2: (a) Ligature in the frontal lower incisor tooth of the rats. (b) Examination of inflammation in the frontal lower incisor tooth of a rat five days post-ligature.
On day 0, after confirmation of periodontitis, the 24 rats were further divided into two groups, six rats in the untreated group (the control group), and another 18 rats were further subdivided into three groups of six animals. Since ligature was left for five days for induction of periodontitis, some rats did not survive due to infection and hence could not be used in the final results. Group 1 received 5% metronidazole-loaded gel, group 2 received 0.15% diclofenac potassium loaded gel, and group 3 received 5% metronidazole and 0.15% diclofenac potassium loaded gel formulation. The gel of 0.2 mL was administered using 1 mL syringe with a 20G needle (Terumo, Japan, Tokyo) to the infected periodontium [Figure 3].

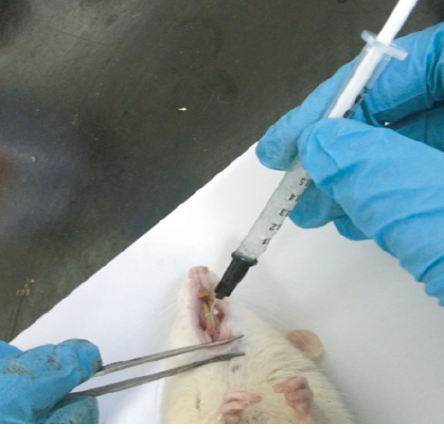 Figure 3: Delivery of gel formulation locally to the infected periodontium of rat.
Figure 3: Delivery of gel formulation locally to the infected periodontium of rat.
After administration of gel, n-butyl-2-cyanoacrylate was applied in a thin layer over the pocket to prevent leakage of gel from the pocket.23,24 On day 7, all rats were euthanized by exposing to ether, and their maxilla excised and examined (Histology Laboratory, Lam Wah Ee Hospital, Penang, Malaysia). The samples were decalcified by 1/1 mixture of 8% formic acid and 8% chlorhydric acid for two weeks. The tissue was trimmed and dehydrated using isopropyl alcohol at different concentrations of 70%, 90%, and 100% clarified in xylene. The tissue was fixed on paraffin block and sectioned before staining with hematoxylin and eosin (H&E). It was examined histologically, and score evaluations were applied under a microscope (Olympus, Germany). The indicators used for evaluation were inflammatory cells count, necrosis, edema, presence of granulation, complete organization of periodontal ligament without tearing, and re-epithelization as the repair parameters of the healing process. Each parameter was scored from 0 to 3, where 0 (0.0%) = normal, 1 (16.6%) = mild increase, 2 (33.3%) = moderate increase, and 3 (50.0%) = severe increase. The histological analysis was performed by a blind examiner in all groups.11,25–27
The results of healing parameters were evaluated using the Kruskal-Wallis test (GraphPad PRISM, Version 5.01, USA). When there was a statistically significant difference, post-hoc Dunn test was performed. A statistically significant difference was considered at p < 0.050.
Results
Table 2 presents the gelation temperature values of various formulations comprising different compositions of poloxamer 407 and 188. Poloxamer content of 30% was selected for subsequent experiments as lower polymer content was employed, and the temperature was close to the physiological temperature of the oral cavity. We used a blend of poloxamer 407 and 188 with xanthan gum.
Table 2: Poloxamer mixtures with corresponding gelation temperature values.
|
7.5 (5.0: 2.5) |
No gelation up to 45 |
|
15.0 (10.0: 5.0) |
No gelation up to 45 |
|
22.5 (15.0: 7.5) |
No gelation up to 45 |
|
30.0 (20.0: 10.0) |
35.7 ± 0.6 |
|
37.5 (25.0: 12.5) |
33.2 ± 0.8 |
|
45.0 (30.0: 15.0) |
30.2 ± 0.8 |
|
52.5 (35.0: 17.5) |
28.0 ± 1.0 |
Mean±SD, N = 3.
Syringability is an important parameter for gel delivered by syringe. The work needed to expel the gel from the syringe was measured, and it needed about 4.3±0.9 (N Sec) to inject smoothly all the gel at the site of application.
Figure 4 shows the mean release profiles of metronidazole and diclofenac potassium of in situ gelling formulation. Metronidazole release was relatively faster than that of diclofenac potassium. It could be observed that the in situ gelling formulation could prolong the release of metronidazole and diclofenac potassium to seven days.

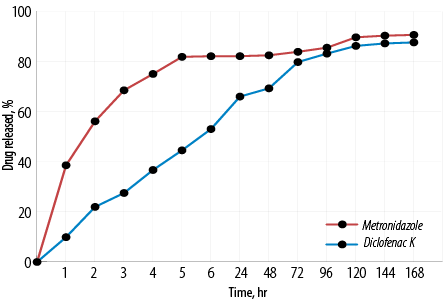 Figure 4: Mean in vitro release curves of metronidazole and diclofenac potassium from in situ gelling formulation. Mean±SD, N = 3.
Figure 4: Mean in vitro release curves of metronidazole and diclofenac potassium from in situ gelling formulation. Mean±SD, N = 3.
Table 1 illustrates the scores of inflammatory parameters of untreated and treated rats after induction of periodontitis. From histopathology examination, the untreated group revealed intense inflammatory response to irritation caused by the ligature. There was an abundance of infiltration of PMN inflammatory cells and monocytes when compared with normal tissues without exposure to LIP [Figure 5].

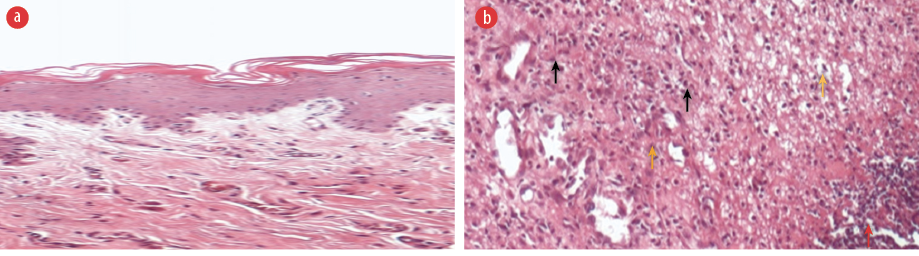 igure 5: Histopathological photomicrographs of periodontal tissues. H&E staining. (a) No induction of periodontitis, magnification = 40 ×. (b) Untreated after ligature-induced periodontitis showing abundant infiltration of inflammatory cells (black arrows) with edema (yellow arrow) and granulation tissue with necrosis (red arrow) poor repair, magnification = 100 ×.
igure 5: Histopathological photomicrographs of periodontal tissues. H&E staining. (a) No induction of periodontitis, magnification = 40 ×. (b) Untreated after ligature-induced periodontitis showing abundant infiltration of inflammatory cells (black arrows) with edema (yellow arrow) and granulation tissue with necrosis (red arrow) poor repair, magnification = 100 ×.
For the group treated with metronidazole loaded gel, we observed inflammatory cells diffused among the gingival epithelial and granulation tissue. Necrosis was less than that observed in the untreated group [Figure 6]. A statistically significant improvement was observed solely for edema between the untreated and the group treated with metronidazole loaded gel.

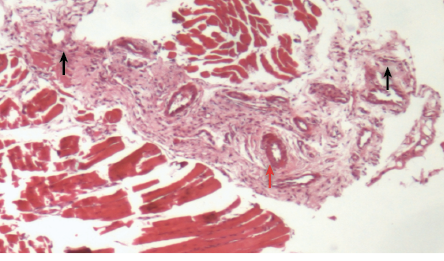 Figure 6: Histopathological photomicrograph of periodontal tissue treated with metronidazole loaded gel formulation showing moderate infiltration of inflammatory cells (black arrows) and granulation tissue (red arrow), magnification = 40 × H&E staining.
Figure 6: Histopathological photomicrograph of periodontal tissue treated with metronidazole loaded gel formulation showing moderate infiltration of inflammatory cells (black arrows) and granulation tissue (red arrow), magnification = 40 × H&E staining.

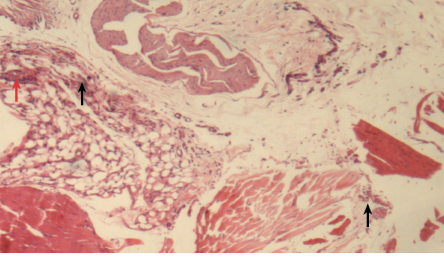 Figure 7: Histopathological photomicrograph of periodontal tissue treated with diclofenac potassium loaded gel formulation showing moderate infiltration of inflammatory cells (black arrows) and granulation (red arrow), magnification = 40 ×. H&E staining.
Figure 7: Histopathological photomicrograph of periodontal tissue treated with diclofenac potassium loaded gel formulation showing moderate infiltration of inflammatory cells (black arrows) and granulation (red arrow), magnification = 40 ×. H&E staining.
Similarly, the group treated with diclofenac potassium loaded gel showed relatively lower scores for all the inflammatory parameters compared with the untreated group [Figure 7]. There was a statistically significant improvement in edema and necrosis when treated with diclofenac potassium loaded gel. There was no statistically significant difference in any inflammatory parameter between the gel loaded with diclofenac potassium and the gel loaded with metronidazole.
Figure 8 shows the healing of the group treated with metronidazole and diclofenac potassium loaded gel was more obvious in terms of improvement and continual organization of periodontal ligament, formation of new epithelial or re-epithelization and absence of edema. Table 1 also shows that treatment with gel loaded with both metronidazole and diclofenac potassium, showed significant improvement in the presence of PMN, re-epithelization, necrosis, and edema.

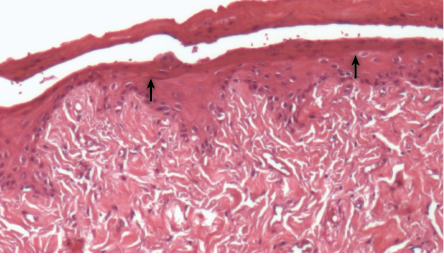 Figure 8: Histopathological photomicrographs of periodontal tissue after treatment with metronidazole and diclofenac potassium loaded gel formulation, showing weak inflammatory response and formation of epithelial layer (black arrows) with normal thickness, hallmarks of the healing process, magnification = 40 ×. H&E staining.
Figure 8: Histopathological photomicrographs of periodontal tissue after treatment with metronidazole and diclofenac potassium loaded gel formulation, showing weak inflammatory response and formation of epithelial layer (black arrows) with normal thickness, hallmarks of the healing process, magnification = 40 ×. H&E staining.
There was a statistically significant difference in the scores of the inflammatory parameters namely, presence of PMN, granulation, re-epithelization, necrosis, and edema between the untreated rats and rats treated with gel formulation loaded with metronidazole, diclofenac potassium, or both metronidazole and diclofenac potassium. Moreover, when gels loaded with a single drug and two drugs were compared, a statistically significant difference was only obtained in re-epithelization and organization of the ligament between gel formulation loaded with diclofenac potassium or metronidazole and loaded with two drugs.
Generally, there was no statistically significant difference in any inflammatory parameter between the gel loaded with diclofenac potassium and the gel loaded with metronidazole separately [Table 1].
Discussion
The physical properties of polymeric solution depend primarily on concentration. However, the physical properties of poloxamer solution are influenced by both concentration and temperature. Poloxamer solution exhibits thermoreversible gelation.28 The temperature-dependent gelation of poloxamer solutions is attributed to the changes in their micellar properties. Incorporation of both poloxamer 407 and 188 could modify the PEO/PPO ratio, leading to changes in gelation temperature. The gelation temperature of 30.0% and 37.5% poloxamer contents were within the temperature of 33–37 °C in the oral cavity, which was the desired gelation temperature range and suitable for periodontal application.29,30 We used a blend of poloxamer 407 and 188 with xanthan gum, which could affect the syringeability and the gelation temperature.
The work of injection/syringeability was more accurate than the simple measurement of force to characterize the extrusion of syringe content.20 Increasing the content of xanthan gum to 2.0% played an important role in syringeability.31,32
It could be observed that the in situ gelling formulation could prolong the release of metronidazole and diclofenac potassium to seven days. This could reduce the dosing frequency and improve patient compliance by administering the formulation once a week.
In histopathological examination, we found that the untreated group revealed intense inflammatory response to irritation caused by the ligature. Edema, necrosis, and granulation could be observed in the tissues. Periodontal ligaments were disorganized. No re-epithelization occurred. The findings were consistent with the results reported in previous studies.23,33
For the group treated with metronidazole loaded gel, inflammatory cells diffused among the gingival epithelial and granulation tissue were present. Necrosis was less than that observed in the untreated group. This observation was consistent with the antibacterial activity of metronidazole against both gram positive and negative microorganisms.11,27,34
Similarly, the group treated with diclofenac potassium loaded gel showed relatively lower scores for all inflammatory parameters compared with the untreated group. This observation indicated that topical therapy of non-steroidal anti-inflammatory drug (NSAID) could reduce periodontitis disease progression via modulation of arachidonic acid metabolism. These results were consistent with the findings of other reported studies.26,35,36 Some studies showed that diclofenac potassium can combat multidrug-resistant bacteria and problematic antibiotic-resistant bacterial infections.37–39
Diclofenac potassium showed a beneficial anti-inflammatory effect, which could give more pronounced healing.40 Diclofenac potassium might compliment the antibacterial action of metronidazole in inhibition of DNA synthesis and eradication of the pathogens.41,42
There was no statistically significant difference in any inflammatory parameter between the gel loaded with diclofenac potassium and the gel loaded with metronidazole. The results revealed that the treatment of periodontal disease could be achieved successfully using a thermosensitive in situ gel delivery system. The use of a combination of NSAID and antibiotic could accelerate the healing process compared to the use of antibiotic or anti-inflammatory drug alone in treating periodontal disease.43 This can be seen from the significant improvement in more inflammatory parameters when both drugs were used.
Conclusion
We successfully developed a thermosensitive, in situ gelling formulation containing metronidazole and diclofenac potassium for the treatment of periodontal disease in a rat model. The gel demonstrated prolonged drug release over an extended period. This local delivery system is anticipated to improve patient compliance and clinical outcome as the gel formulation could be applied directly to the site of action. The effectiveness of the co-therapy of antibacterial and anti-inflammatory agents such as metronidazole and diclofenac potassium can play a potentially advantageous role in the clinical therapy of periodontal disease, which is more effective than using either antibacterial or anti-inflammatory drugs. The co-therapy could add benefits towards the eradication of chronic periodontal disease. This rationalizes the prescribing treatment of the combination of antibacterial and anti-inflammatory agents.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Arigbede AO, Babatope BO, Bamidele MK. Periodontitis and systemic diseases: a literature review. J Indian Soc Periodontol 2012 Oct;16(4):487-491.
- 2. Wade WG. The oral microbiome in health and disease. Pharmacol Res 2013 Mar;69(1):137-143.
- 3. Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med 2019 Jul;8(8):1135.
- 4. Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1-year results. J Clin Periodontol 2007 Mar;34(3):243-253.
- 5. Johnson ML, Uhrich KE. Concurrent release of admixed antimicrobials and salicylic acid from salicylate-based poly(anhydride-esters). J Biomed Mater Res A 2009 Dec;91(3):671-678.
- 6. Karthikeyan K, Durgadevi R, Saravanan K, Shivsankar K, Usha S, Saravanan M. Formulation of bioadhesive carbomer gel incorporating drug-loaded gelatin microspheres for periodontal therapy. Trop J Pharm Res 2012;11(3):335-343 .
- 7. Johnson A, Sprangers A, Cassidy P, Heyrman S, Hinshaw JL, Lubner M, et al. Design and validation of a thermoreversible material for percutaneous tissue hydrodissection. J Biomed Mater Res B Appl Biomater 2013 Nov;101(8):1400-1409.
- 8. Beg S, Dhiman S, Sharma T, Jain A, Sharma RK, Jain A, et al. Stimuli responsive in situ gelling systems loaded with PLGA nanoparticles of moxifloxacin hydrochloride for effective treatment of periodontitis. AAPS PharmSciTech 2020 Jan;21(3):76.
- 9. Gomes EW, Casarin M, Martins TM, da Silva AF. Local delivery therapies as adjuvants to non-surgical periodontal treatment of periodontitis grade C: a systematic review. Clin Oral Investig 2020 Dec;24(12):4213-4224.
- 10. Wang B, Booij-Vrieling HE, Bronkhorst EM, Shao J, Kouwer PH, Jansen JA, et al. Antimicrobial and anti-inflammatory thermo-reversible hydrogel for periodontal delivery. Acta Biomater 2020 Oct;116:259-267.
- 11. Liu R, Li N, Liu N, Zhou X, Dong ZM, Wen XJ, et al. Effects of systemic ornidazole, systemic and local compound ornidazole and pefloxacin mesylate on experimental periodontitis in rats. Med Sci Monit 2012 Mar;18(3):BR95-BR102.
- 12. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol 2008 Aug;79(8)(Suppl):1585-1591.
- 13. Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol 1998 Jan;160(1):403-409.
- 14. Oz HS, Puleo DA. Animal models for periodontal disease. Journal of Biomedicine and Biotechnology. 2011 Oct;2011.
- 15. Tamaddon AM, Saadati F, Khosropanah H, Dehghani Nazhvani A, Yousefi GH. Histologic evaluation of new doxycycline gel formulation for subgingival application in experimental periodontitis in rats. International Journal of Scientific Research in Dental and Medical Sciences. 2020 Dec;2(4):107-114.
- 16. Jones DS, Woolfson AD, Brown AF, O’Neill MJ. Mucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: in vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J Control Release 1997 Nov;49(1):71-79.
- 17. Borolr Orloe DK, Sriharsha SN. Yashwant Kumar D, Neelim KSSN. Preparation and characterization of metronidazole and minocycline HCl mucoadhesive gel for the treatment of periodontitis. International Journal of Chemistry and Pharmaceutical Sciences. 2014;2(12):1344-1350.
- 18. Senyiğit ZA, Karavana SY, Eraç B, Gürsel O, Limoncu MH, Baloğlu E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm 2014 Jun;64(2):139-156.
- 19. Nida’a MA, Shalaby K, Khiang PK, and Ahuja A. Development and evaluation a simultaneous assay method of metronidazole and diclofenac potassium in pharmaceutical formulation. Canadian Journal of Pure and Applied Sciences 2013 Aug:2333-2339.
- 20. Zhang Q, Fassihi MA, Fassihi R. Delivery considerations of highly viscous polymeric fluids mimicking concentrated biopharmaceuticals:assessment of injectability via measurement of total work done “WT”. AAPS PharmSciTech 2018 May;19(4):1520-1528.
- 21. Sagar P, Prasad K, Lalitha RM, Ranganath K. Cyanoacrylate for intraoral wound closure: a possibility? Int J Biomater 2015;2015:165428.
- 22. Fernandes LA, Martins TM, Almeida JM, Nagata MJ, Theodoro LH, Garcia VG, et al. Experimental periodontal disease treatment by subgingival irrigation with tetracycline hydrochloride in rats. J Appl Oral Sci 2010 Dec;18(6):635-640.
- 23. Ionel A, Lucaciu O, Moga M, Buhatel D, Ilea A, Tabaran F, et al. Periodontal disease induced in Wistar rats-experimental study. Human and Veterinary Medicine. 2015 Jun;7(2):90-95.
- 24. Hu Y, Zhang X, Zhang J, Xia X, Li H, Qiu C, et al. Activated STAT3 signaling pathway by ligature-induced periodontitis could contribute to neuroinflammation and cognitive impairment in rats. J Neuroinflammation 2021 Mar;18(1):80.
- 25. Eskandari MM, Ozturk OG, Eskandari HG, Balli E, Yilmaz C. Cyanoacrylate adhesive provides efficient local drug delivery. Clinical Orthopaedics and Related Research (1976-2007). 2006 Oct 1;451:242-50.
- 26. Botelho MA, Martins JG, Ruela RS, Queiroz DB, Ruela WS. Nanotechnology in ligature-induced periodontitis: protective effect of a doxycycline gel with nanoparticules. J Appl Oral Sci 2010 Jul-Aug;18(4):335-342.
- 27. Ramadan E, Borg TM, El Hawary YM, Saleh N. Formulation and evaluation of metronidazole bioadhesive matrices for treatment of periodontitis. Bulletin of Pharmaceutical Sciences. Assiut 2010 Jun;33(1):79-94.
- 28. Yong CS, Sah H, Jahng Y, Chang HW, Son JK, Lee SH, et al. Physicochemical characterization of diclofenac sodium-loaded poloxamer gel as a rectal delivery system with fast absorption. Drug Dev Ind Pharm 2003 May;29(5):545-553.
- 29. Choi HG, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm 1998 Apr;165(1):33-44.
- 30. Baloglu E, Karavana SY, Senyigit ZA, Hilmioglu-Polat S, Metin DY, Zekioglu O, et al. In-situ gel formulations of econazole nitrate: preparation and in-vitro and in-vivo evaluation. J Pharm Pharmacol 2011 Oct;63(10):1274-1282.
- 31. Karavana SY. A new in-situ gel formulation of itraconazole for vaginal administration. Pharmacol Pharm 2012 Oct;3(04):417.
- 32. Singh KP, Chhabra G, Sharma V, Pathak K. Thermosensitive periodontal sol of ciprofloxacin hydrochloride and serratiopeptidase: pharmaceutical and mechanical analysis. Int J Pharm Investig 2014 Jan;4(1):5-14.
- 33. Botelho MA, Paixao MS, Allegretti C, Argentino-Júnior J, Araujo R, Rodrigues K, et al. Anti-inflammatory effect of diclofenac diethylammonium gel on acute phase of ligature induced periodontitis in rats. Lat Am J Pharm 2010 Dec;29(8):1371-1376.
- 34. Sander L, Frandsen EV, Arnbjerg D, Warrer K, Karring T. Effect of local metronidazole application on periodontal healing following guided tissue regeneration. Clinical findings. J Periodontol 1994 Oct;65(10):914-920.
- 35. Brägger U, Mühle T, Fourmousis I, Lang NP, Mombelli A. Effect of the NSAID flurbiprofen on remodelling after periodontal surgery. J Periodontal Res 1997 Oct;32(7):575-582.
- 36. Paquette DW. Topical (S)-ketoprofen and the treatment of adult periodontitis. J Dent Res 1998;77:2953.
- 37. Dutta NK, Annadurai S, Mazumdar K, Dastidar SG, Kristiansen JE, Molnar J, et al. Potential management of resistant microbial infections with a novel non-antibiotic: the anti-inflammatory drug diclofenac sodium. Int J Antimicrob Agents 2007 Sep;30(3):242-249.
- 38. Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol 2013 Mar;31(3):177-184.
- 39. Salem-Milani A, Balaei-Gajan E, Rahimi S, Moosavi Z, Abdollahi A, Zakeri-Milani P, et al. Antibacterial effect of diclofenac sodium on Enterococcus faecalis. Journal of dentistry (Tehran, Iran) 2013;10(1):16.
- 40. Cavagni J, Muniz FW, Rosing CK. The effect of inflammatory response modulator agents on gingivitis and periodontitis. RGO Rev Gaúch Odontol 2016 Jul;64:312-319.
- 41. Hersh EV, Hammond BF, Fleury AA. Antimicrobial activity of flurbiprofen and ibuprofen in vitro against six common periodontal pathogens. J Clin Dent 1991;3(1):1-5.
- 42. Dastidar SG, Ganguly K, Chaudhuri K, Chakrabarty AN. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int J Antimicrob Agents 2000 Apr;14(3):249-251.
- 43. Mohiuddin K, Ravindra S, Ahmed MG, Murthy S, Smitha BR. Single use of tetracycline with and without diclofenac sodium as local drug delivery in pocket therapy: a clinico-microbiological study. J Investig Clin Dent 2011 Nov;2(4):280-286.
