Vascular anomalies are soft tissue lesions that affect up to 10% of newborns and were first classified by Mulliken and Glowacki in 1982 into congenital vascular malformation (CVM) and vascular tumors.1–3 Despite the advancements in this field, CVMs remain a diagnostic and therapeutic challenge to the treating physicians; therefore, requiring a multidisciplinary approach.4
Case reports
Case one
An eight-year-old girl born to consanguineous parents presented with a two-year history of on and off proptosis, redness, and pain of the right eye [Figure 1a]. These symptoms were provoked by an upper respiratory tract infection – three episodes in two years. Magnetic resonance imaging (MRI) done at initial presentation showed a lymphovenous right eye CVM [Figure 1b]. The patient was managed with intravenous steroids and sclerotherapy, followed by eye drops (brinzolamide+timolol). Follow-up MRI showed regression, and she has been asymptomatic for three years.
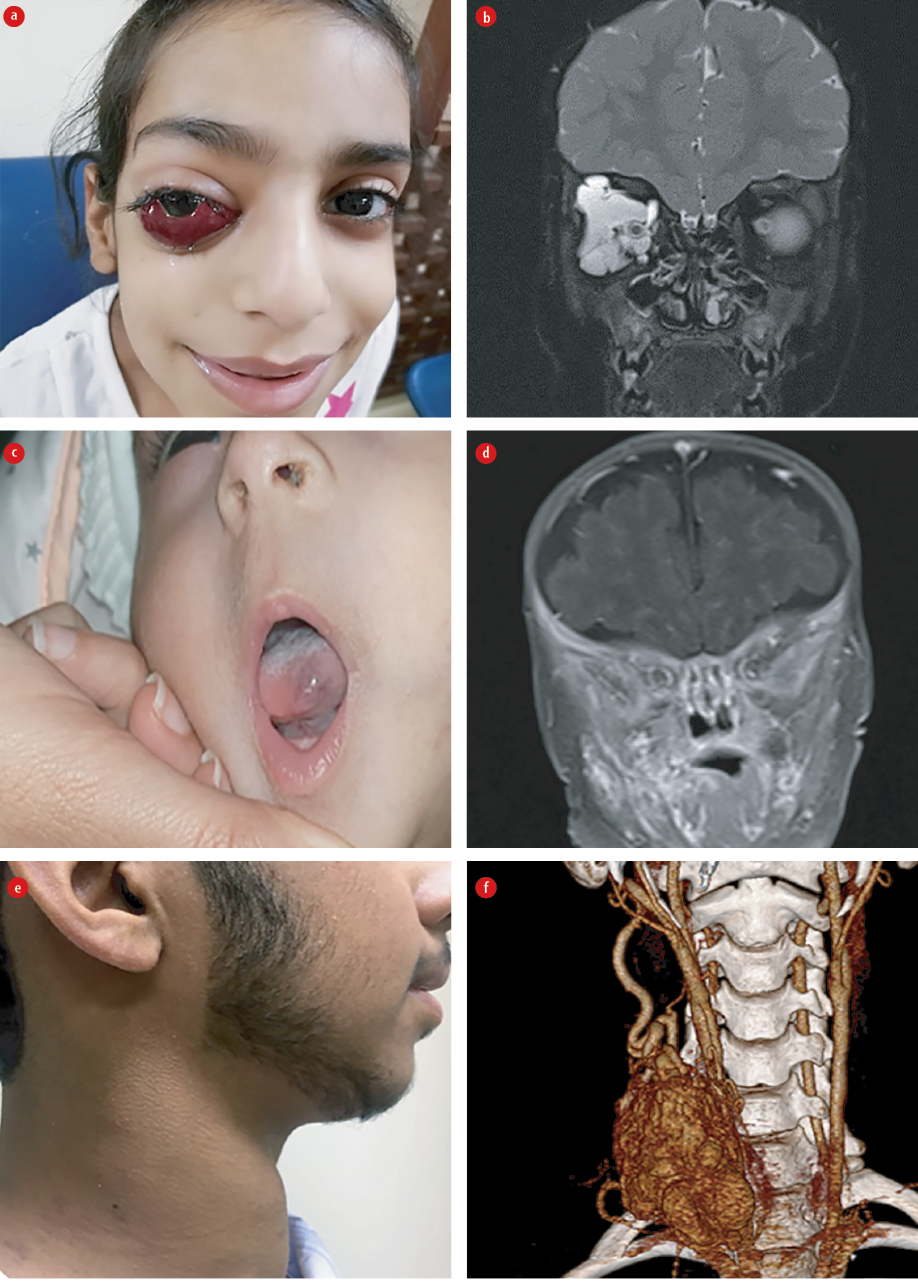
Figure 1: (a) Lymphovenous congenital vascular malformation (CVM), right eye. (b) MRI head; lymphovenous CVM, right eye. (c) Venous malformation (VM) of the right cheek and the tongue. (d) MRI head, VM, right side of the face. (e) Right lower neck pulsatile mass. (f) CT angiogram;
high flow arteriovenous malformation .
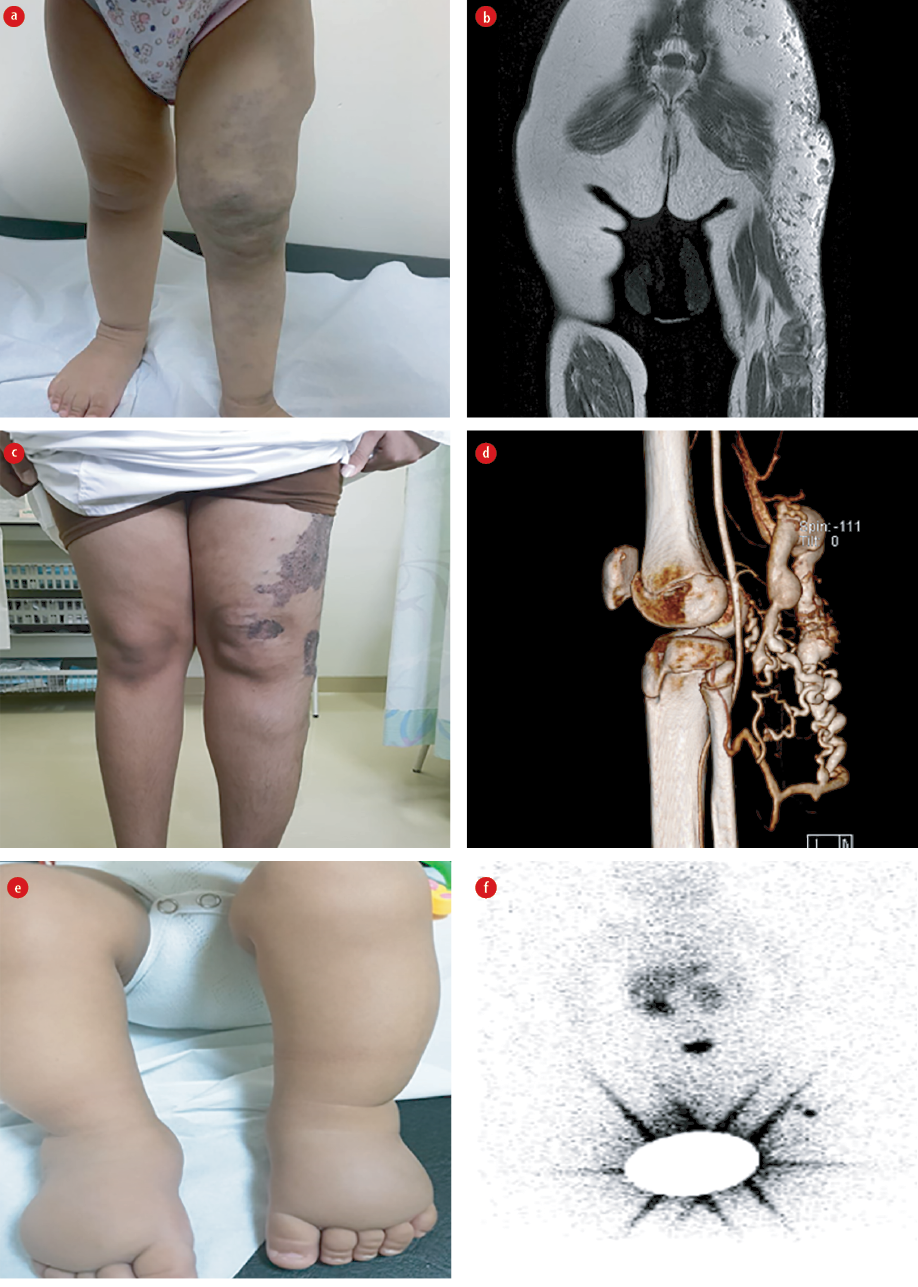
Figure 2: (a) Left lower limb Klippel-Trennauay syndrome (KTS); limb hypertrophy. (b) MRI lower limb showing; capillary, venous, and lymphatic malformations; KTS. (c) Angiomatous lesion of the left thigh; Park–Weber Syndrome. (d) MRI lower limbs; left thigh capillary malformation and AVF. (e) Primary congenital lymphedema of lower limbs. (f) Lymphoscintigraphy; no uptake.
Table 1: Summary of patients’ gender, age, diagnosis, imaging findings, and management.
|
1 |
8 years |
Female |
Rt eye lympho venous malformation |
MRI |
Large lobulated insinuating lesion noted within the coronal compartment of the right orbit. |
Intravenous steroids, sclerotherapy, followed by eye drops (brinzolamide+timolol). |
|
2 |
4 months |
Male |
VM of the rt cheek and tongue |
MRI |
Multi-spatial heterogeneous ill-defined lesion along the right side of the tongue and cheek. |
Conservative. If symptoms of pain or bleeding develop, sclerotherapy would be offered. |
|
3 |
14 years |
Male |
AVM of the rt lower neck |
CT angiogram |
High flow AVM multiple feeders from the external carotid artery, thyrocervical trunk, and the vertebral artery draining into the subclavian and internal jugular vein. |
Five sessions of angioembolization using glue and onyx injection, planned for surgical. |
|
4 |
15 months |
Female |
KTS, left lower extremity |
MRI |
Capillary, venous, lymphatic malformations of the left lower extremity. |
Conservative; custom-made grade 2 compression hosiery for the affected limb up to the waist and height correction foot wear. |
|
5 |
15 years |
Male |
PWS, left lower extremity |
MRI
angiography |
Arteriovenous fistula. |
Two sessions of glue embolization; above-knee grade 2 compression stockings. |
MRI: magnetic resonance imaging; CVM: congenital vascular malformation; KTS: Klippel-Trennauay syndrome; LM: lymphatic malformation;
AVM: arteriovenous malformation.
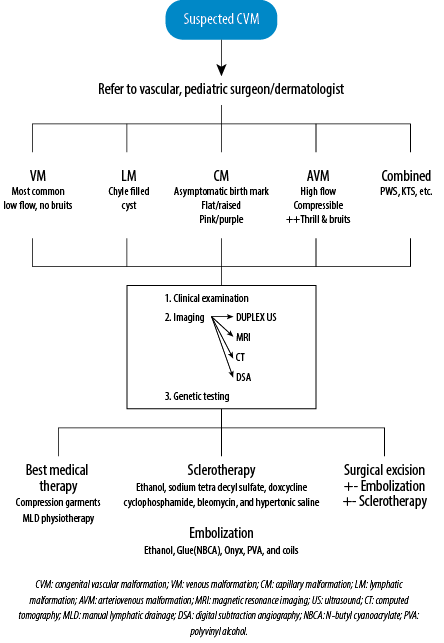
Figure 3: Diagnostic and treatment algorithm of CVMs.
Case two
A four-month-old girl diagnosed with multiple development anomalies – atrial septal defect (3.5 mm), pulmonary stenosis, subtle dysmorphism, developmental dysplasia of the hip, and generalized tonic clonic seizure, presented with bluish discoloration of the skin around the cheek and tip of the tongue since birth, with multiple venous malformation (VM) on the right side of cheek, which become more prominent when the child cried [Figure 1c]. MRI at initial presentation showed thrombosis of the right transverse and sigmoid venous sinuses with multi-spatial heterogeneous ill-defined lesion along the right side of the tongue and cheek representing a VM [Figure 1d]. The patient is on a daily dose of enoxaparin 6.5 mg for cerebral venous thrombosis. If pain or bleeding develops, sclerotherapy would be offered for the VM.
Case three
A 14-year-old boy presented with a three-year history of right lower neck swelling that increased in size gradually was pulsatile and associated with mild pain. He had no history of trauma. Examination revealed a 12 × 10 cm non-tender, pulsatile swelling at the carotid triangle with engorged veins, a thrill, and bruit [Figure 1e]. A computed tomography angiogram [Figure 1f] confirmed a high flow arteriovenous malformation (AVM), with multiple feeders from the external carotid artery, thyrocervical trunk, and the vertebral artery draining into the subclavian and internal jugular veins. An echocardiography showed left ventricular hypertrophy in keeping with the high flow AVM. He underwent five sessions of angioembolization using glue and onyx injection and is planned for surgical excision.
Case four
A 15-month-old girl presented with an irregular blue patch on her left lower extremity since birth, with hemihypertrophy of the limb [Figure 2a]. She started to walk with a limp. The Galeazzi test is positive for the discrepancy between the tibia. A diagnosis of Klippel-Trennauay syndrome (KTS) was made based on the presence of capillary, venous, lymphatic malformations seen on MRI [Figure 2b]. We advised custom-made grade 2 compression hosiery for the affected limb up to the waist and height correction footwear. The child will be followed-up regularly, and imaging is planned once she is 18 years old or earlier if she develops any complications (e.g., bleed, thrombosis, or infection).
Case five
A 15-year-old boy presented with left lower limb swelling with an overlying angiomatous lesion in the thigh [Figure 2c]. He was treated as a case of KTS. He underwent radiofrequency ablation, sclerotherapy, and attempted varicose vein surgery abroad. Unfortunately, during the excision of varicose veins over the popliteal fossa, he had significant intraoperative bleeding that required blood transfusions, and the surgery was abandoned. MRI [Figure 2d] and angiography showed the presence of arteriovenous fistula consistent with Park–Weber Syndrome (PWS). Following two sessions of glue embolization of the fistulas, he had a reduction in edema and pain. Above-knee grade 2 compression stockings continue to be used.
Case six
An eight-month-old boy born to double consanguineous parents presented with swelling of both feet since birth. On examination, he had bilateral pitting edema confined to the dorsal aspect of both feet [Figure 2e]. Lymphoscintigraphy suggested primary congenital lymphedema as there was no tracer uptake [Figure 2f]. Custom-made grade 2 below-knee stockings were prescribed, and his parents were taught manual lymphatic drainage.Table 1 gives a summary of the six cases.
Discussion
CVMs result from an arrest in the development of blood vessels during embryogenesis. Cells of mesodermal origin that arrest in the early stages of embryogenesis develop into extratruncular CVM and manifest following a triggering factor (e.g., menarche, pregnancy, trauma, or surgery). These lesions can recur after treatment. On the other hand, truncular CVMs arise when embryogenesis arrests during the formation of the vascular trunk and tend to grow with the child, have minimal risk of recurrence, and can be associated with hemodynamic compromise.5 Mulliken and Glowacki classified vascular anomalies into CVM and vascular tumors. However, this classification was revised in 1997 by the International Society for the Study of Vascular Anomalies (ISSVA). Modified Hamburg classification subdivides CVM into capillary, venous, lymphatic, arteriovenous, and combined malformations [Figure 3]. Name-based eponyms have been used for many years, such as KTS, PWS, Servelle–Martorell syndrome, etc. 5–9
Seventy percent of VMs are considered the most common CVM, followed by lymphatic malformations (12%), AVMs (8%), combined malformation syndromes (6%), and capillary malformations (4%). The majority of these lesions are sporadic. The importance of family history cannot be understated.5–7
Conclusion
CVM in the Omani population is not as uncommon as thought to be. Knowing the different clinical features, characteristic imaging findings, and management options of these lesions would help healthcare professionals diagnose, counsel, and refer appropriately. Molecular genetic studies can be conducted in patients with a family history of CVM. A multidisciplinary team is needed to treat these patients holistically, and a national registry should be started.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from the patients’ parents.
Acknowledgements
We thank the parents for permitting us to take the pictures of the patients and publish them.
references
- 1. Cabrera J, Cabrera J Jr, García-Olmedo MA, Redondo P. Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol 2003 Nov;139(11):1409-1416.
- 2. Nassiri N, Cirillo-Penn NC, Thomas J. Evaluation and management of congenital peripheral arteriovenous malformations. J Vasc Surg 2015 Dec;62(6):1667-1676.
- 3. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis 2010 Jul;16(5):405-418.
- 4. Mulligan PR, Prajapati HJ, Martin LG, Patel TH. Vascular anomalies: classification, imaging characteristics and implications for interventional radiology treatment approaches. Br J Radiol 2014 Mar;87(1035):20130392.
- 5. Lee BB, Laredo J. Coagulation issue in venous malformation and its management. In: GHR Rao, E Kalodiki, WA Leong, J Fareed, editors. Clinical handbook of management of antithrombotic & thrombolytic therapy. New Delhi, India: Kontentworx; 2014. p. 93-127.
- 6. Kapadia S, Thakore V, Patel H. Vascular malformations: an update on classification, clinical features, and management principles. Indian Journal of Vascular and Endovascular Surgery. 2017;4(4):152-162.
- 7. Adams DM, Brandão LR, Peterman CM, Gupta A, Patel M, Fishman S, et al. Vascular anomaly cases for the pediatric hematologist oncologists-An interdisciplinary review. Pediatr Blood Cancer 2018 Jan;65(1):e26716.
- 8. Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics 2015 Jul;136(1):e203-e214.
- 9. Premkumar P, Stephen E, John JM, Kota AA, Samuel V, Selvaraj D, et al. Management of Klippel-trenaunay syndrome from a single center in India: experience shared. Indian Journal of Vascular and Endovascular Surgery 2018;5(3):149-153.