Over the past decade, advanced neonatal intensive care has remarkably improved the survival rates of premature infants. However, prematurity and infectious diseases continue to be notable reasons for infant morbidity and mortality.1,2 Neurodevelopmental outcomes for the surviving preterm infants—especially those with very low birth weights (LBW)—are still suboptimal. Sepsis is an established contributor to poor neurodevelopmental outcomes in preterm neonates. Late-onset sepsis (LOS)3 has been reported in 20%–50% premature neonates. Prevalence is even higher among neonates with lower gestational age and birth weight.4,5 Such high susceptibility to neonatal intensive care unit (NICU)-induced LOS is attributed to factors such as prolonged hospital stay, invasive procedures like intubation and central line insertions, delayed enteral feed, and early exposure to broad spectrum antibiotics.1,6,7
Most (85%) nosocomial infections contracted in the NICU and the subsequent LOS are caused by gram-positive bacteria, mainly (55%) coagulase-negative Staphylococci (CONS).8 Gram-negative infections also result in significant neonatal morbidity and mortality. There is also a risk of meningitis if the LOS pathogen spreads via the hematogenous route (through the choroid plexus) or directly from an open wound, central line, fetal scalp monitor, or defective neural tube.9
Most brain maturation and neurological development occur in the last trimester of pregnancy, continuing up to the early postnatal years.10 In a preterm neonate, the immature brain, especially the oligodendrocytes lineage, is extremely vulnerable to a hypoxic-ischemic insult, hemorrhage, or a systemic inflammatory reaction against an infection irrespective of its origin, whether maternofetal or NICU-acquired. Several mechanisms, such as an endotoxin-induced cytokine storm, disturbed auto-regulation, excitotoxicity, and inadequate perfusion, have been proposed for the pathogenesis of sepsis that damages the immature brain. An association between intraventricular hemorrhage (IVH) or periventricular white matter lesions and the release of IL-6 (a pro-inflammatory cytokine) resulting from intrauterine infection has been reported, substantiating the hypothesis of a similar course postnatally during LOS.11 Further, it is known that preterm infants with LOS, even without meningitis, have considerable risk of poorer neuro-developmental outcomes.12 Similarly, gastrointestinal infections like necrotizing enterocolitis (NEC) may trigger systemic inflammatory response resulting in significant neuronal injury with neurodevelopmental implications visible at 18–22 months follow-up.7,13–16
Neuroimaging is an important diagnostic tool in ensuring competent NICU care and determining prognosis in case of neonatal comorbidities.17 A preterm neonate’s brain is extremely vulnerable to IVH and white matter injury (WMI). Magnetic resonance imaging (MRI) helps localize lesions and may provide cues for possible motor and cognitive deficits that may manifest with age.18
Though multiple clinical studies have shown that neonatal sepsis increases the risk of WMI and IVH,19–21 data is scarce regarding the link between sepsis and brain injury. The present study seeks to use MRI data to evaluate the link between late neonatal sepsis and brain injury and possible neurodevelopmental outcomes by three years of age.
Methods
We retrospectively analyzed the medical records of all infants with gestational age 24–32 weeks (excluding those with congenital anomalies or syndromes) admitted to a level-III NICU at the Al-Sabah Maternity Hospital, Kuwait, from January to December 2017. The study was approved by the Ethics Committee, Ministry of Health, Kuwait (20171420).
The baseline characteristics for all patients were collected from their medical charts. Neonatal sepsis was confirmed using a positive culture, which was defined as any sample of blood or cerebrospinal fluid which tested positive for bacterial growth. A blood culture was considered contaminated if the presence of gram-positive cocci in it was negated by another culture drawn 30-minutes apart. Early-onset sepsis (EOS) was described by a positive blood culture occurring ≤ 72 hours after birth, and LOS as an infection contracted after this period.22 A case of sepsis was considered severe if associated with hemodynamic instability and disseminated intravascular coagulation (DIC).
Systemic hypotension was characterized by systolic or diastolic or mean blood pressure below the 3rd centile for age and needing inotropic support. NEC was categorized using Bell’s criteria (stage ≥ 2).23 Patent ductus arteriosus (PDA) was diagnosed based on echocardiographic evidence of a hemodynamically significant PDA requiring pharmacological or surgical treatments. Bronchopulmonary dysplasia (BPD) or chronic lung disease (CLD) was designated when supplemental oxygen was required by postnatal day 28 / week 36 postmenstrual age.24 Severe retinopathy of prematurity (ROP) grades 3 and 4 were defined using the International Classification of Retinopathy of Prematurity.25
A brain MRI (1.5 or 3 Tesla) was performed in 181 neonates after swaddling, feeding, or sedation with 25–50 mg/kg chloral hydrate. Besides the standard T1 and T2 weighted images, fluid-attenuated inversion recovery (FLAIR), apparent diffusion coefficient, and diffusion-weighted image sequences were obtained and interpreted by an experienced neuroradiologist. The Papile system was used to grade IVH.26 WMI was categorized according to Miller’s scoring system into mild (< 3 areas of abnormal T1 signal intensity), moderate (> 3 areas of abnormal T1 signal intensity and < 5% hemispheric involvement), and severe (> 5% of the hemisphere involved).18 The presence of cerebellar hemorrhage was also recorded.
Neurological development by 36 months CA was evaluated using the Bayley scales of infant and toddler development-III (BSID-III) cognitive, language, and motor composite scores.27 A ‘moderate’ developmental delay was designated to a worst composite score of 70–84 in ≥ 1 of the 3 domains. Whereas, a score of < 70 for any of the 3 domains, or when unable to assign a score owing to severe mental deficiency or cerebral palsy (CP) (appraised using the Gross Motor Function Classification System (GMFCS) was termed as ‘severe’ delay.28 The GMFCS evaluates the gross motor function of children and youth with CP considering their ability to initiate basic movements like sitting and ambulation (walking or wheeled mobility).
Descriptive statistics were calculated for all data: median, IQR for continuous variables, and frequency (%) for categorical variables. Fisher’s exact test for qualitative and Kruskal–Wallis test for quantitative data were used to compare baseline clinical and imaging characteristics of the 3 study groups. Correlation between sepsis and other clinical variables relevant to brain injury and neurodevelopmental outcomes was computed using univariate linear regression. Similarly, multivariate analysis was done to predict the possible outcomes at 36-month and compare the prognoses for early and LOS cases. The association between infections and their 36-month outcomes was calculated using both unadjusted and adjusted β coefficients. All analyses were performed using Stata/IC 14.2 (Stata Corp, College Station, Texas) and a p-value < 0.050 indicated statistical significance.
Results
Out of 203 preterm neonates in NICU with 24–32 weeks gestational age, 93 (45.8%) remained sepsis-free, 16 (7.9%) had EOS, and 94 (46.3%) developed a LOS [Table 1]. Within the LOS group, 41/94 (43.6%) had histological evidence of chorioamnionitis. The LOS group had a lower median birth weight (0.84 kg; IQR = 0.69–0.97 kg; p < 0.001) versus > 1.00 kg for other groups. The LOS group also had lower gestational age (p < 0.001), length (p < 0.001), and head circumference (p < 0.001). Neonates in this group were more likely to develop PDA, NEC stage ≥ 2, CLD, and ROP (p < 0.001). Klebsiella pneumonia (31.3%) was the commonest organism causing EOS, followed by Streptococcus agalactia (25.0%) and Escherichia coli (18.8%). Among the LOS group, K. pneumonia (57.4%) had the highest prevalence, followed by other pathogens which include CONS, S. epidermidis, Methicillin-resistant, S. aureus, and Serratia (27.7%).
Table 1: Clinical characteristics and MRI findings of the neonates compared by the onset of infection.
|
Antenatal steroid |
83 (89.2) |
12 (75.0) |
85 (90.4) |
0.458 |
|
Histological chorioamnionitis (n = 74) |
24 (25.8) |
9 (56.3) |
41 (43.6) |
0.010 |
|
Gestational age (weeks), median (IQR) |
29.5 (28.1–31.4) |
27.0 (25.8–28.7) |
25.9 (25.0–27.7) |
< 0.001 |
|
Birth weight (grams), median (IQR) |
1190 (1020–1380) |
1045 (832–1335) |
840 (695–966) |
< 0.001 |
|
Head circumference at birth (cm), median (IQR) |
27.0 (25.5–28.0) |
24.0 (23.5–26.0) |
24.0 (22.5–26.0) |
< 0.001 |
|
Length at birth (cm), median (IQR) |
38.0 (36.0–40.0) |
35.0 (33.0–38.5) |
34.0 (31.5–37.0) |
< 0.001 |
|
Male |
48 (51.6) |
8 (50.0) |
52 (55.3) |
0.369 |
|
10 min Apgar score, median (IQR) |
8 (7–9) |
8 (7–9) |
8 (7–8) |
0.090 |
|
PDA |
25 (26.9) |
10 (62.5) |
60 (63.8) |
< 0.001 |
|
NEC stage ≥ 2 |
9 (9.7) |
4 (25.0) |
32 (34.0) |
< 0.001 |
|
ROP |
20 (21.5) |
10 (62.5) |
63 (67.0) |
< 0.001 |
|
CLD |
5 (5.4) |
1 (6.3) |
31 (33.0) |
< 0.001 |
|
Hypotension |
17 (18.3) |
5 (31.3) |
58 (61.7) |
< 0.001 |
|
Severe sepsis |
0 (0.0) |
4 (25.0) |
52 (55.3) |
< 0.001 |
|
Meningitis |
0 (0.0) |
0 (0.0) |
2 (2.1) |
0.574 |
|
White matter injury, n = 181 |
|
|
|
|
|
Mild |
8 (8.6) |
2 (13.3) |
8 (8.5) |
0.586 |
|
Moderate |
7 (7.5) |
3 (18.8) |
5(5.3) |
|
|
Severe |
4 (4.3) |
1 (6.3) |
4 (4.3) |
|
|
IVH |
|
|
|
|
|
Grade I |
3 (3.2) |
4 (25.0) |
5 (5.3) |
|
|
Grade II |
23 (24.7) |
4 (25.0) |
22 (23.4) |
|
|
Grade III |
2 (2.2) |
0 (0.0) |
5 (5.3%) |
|
|
IVH grade III–IV |
25 (26.9) |
4 (25.0) |
27 (28.7) |
0.595 |
|
Cerebellar hemorrhage |
5 (5.4) |
3 (18.8) |
23 (24.5) |
< 0.001 |
|
Organisms |
|
|
|
|
|
Klebsiella pneumonia |
0 (0.0) |
5 (31.3) |
54 (57.4) |
0.010 |
|
Escherichia coli |
0 (0.0) |
3 (18.8) |
6 (6.4) |
0.452 |
|
Enterococcus faecalis |
0 (0.0) |
2 (12.5) |
4 (4.3) |
0.310 |
|
Candida |
0 (0.0) |
0 (0.0) |
3 (3.2) |
0.060 |
|
Streptococcus agalactiae |
0 (0.0) |
4 (25.0) |
1 (1.0) |
0.001 |
PDA: Patent ductus arteriosus; NEC: necrotizing enterocolitis; ROP: Severe retinopathy of prematurity ; CLD: chronic lung disease ; IVH: intraventricular hemorrhage.
data were given as n (%) unless otherwise stated.
Table 2: Risk of cerebellar hemorrhage between organisms.
|
Klebsiella Pneumonia |
43 (28.7) |
18 (58.1) |
0.003 |
|
Escherichia coli |
13 (8.7) |
1 (3.2) |
0.471 |
|
Enterococcus faecalis |
10 (6.7) |
7 (22.6) |
0.012 |
|
Candida |
6 (4.0) |
4 (12.9) |
0.069 |
|
Streptococcus agalactiae |
9 (6.0) |
5 (16.1) |
0.067 |
Others include coagulase-negative Staphylococcus, Staphylococcus epidermidis, methicillin-resistant Staphylococcus aureus, and Serratia marcescens.
Table 3: Bayley-III neurodevelopmental outcome at 36 months corrected age.
|
Bayley-III motor composite score, median (IQR) |
107 (100–115) |
107 (97–110) |
97 (82–107) |
< 0.001 |
|
Bayley-III cognitive composite score, median (IQR) |
103 (100–110) |
105 (100–105) |
100 (90–105) |
0.027 |
|
Bayley-III language composite score, median (IQR) |
110.5 (103–118) |
112 (106–115) |
106 (94–115) |
0.044 |
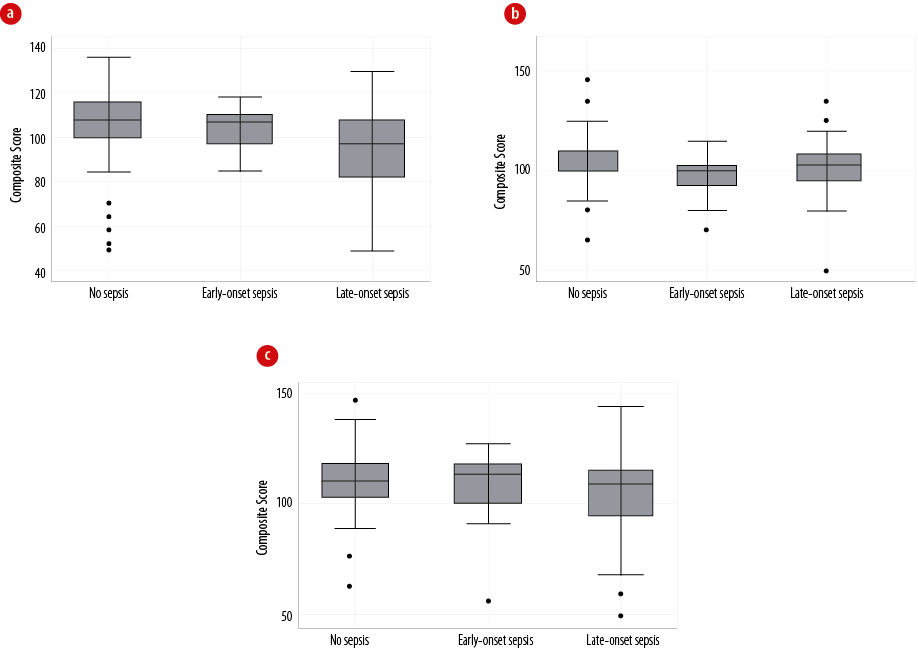
Figure 1: Comparison of Bayley-III developmental outcomes at 36 months corrected age, of no neonatal sepsis, early-onset sepsis, and late-onset sepsis. (a) Motor outcome, (b) cognitive outcome, and (c) language outcome. The vertical line reflects the median and the box indicates the interquartile range. The outliers are indicated by the little black circles.
Table 4: Unadjusted and adjusted β coefficient value for Bayley-III scores at 36 months corrected age in early and late-onset sepsis
|
Bayley-III motor composite score |
*–4.1 (–14.4 to 6.2); 0.435 |
*–6.7 (–11.6 to –1.7); 0.008 |
| |
**–0.1 (–7.7 to 7.5); 0.938 |
**–9.5 (–16.4 to –2.7); 0.007 |
|
Bayley-III cognitive composite score |
*0.8 (–5.1 to 6.8); 0.784 |
*–4.7 (–9.2 to –0.1); 0.046 |
| |
** 2.0 (–5.0 to 9.1); 0.569 |
**–4.7 (–11.2 to 1.7); 0.146 |
|
Bayley-III language composite score |
*–4.1 (–14.8 to 6.5); 0.443 |
*–5.9 (–11.5 to –0.32); 0.038 |
*unadjusted, ** adjusted β (95% CI); p-value.
A total of 181/203 neonates underwent MRI brain at median gestational age of 34 weeks (IQR = 33–36 weeks). Of 203, 18 (8.9%) did not survive. These 18 were from the 22 neonates who did not undergo MRI, while the parents of the four neonates did not consent for MRI. Among the 18 who expired, five had no sepsis, 10 had LOS from Klebsiella infection, two from E. coli, and one from Enterococcus faecalis. WMI and IVH were observed in 42/181 (23.2%) and 68/181 (37.5%) infants respectively. There were no group-wise differences in the severity of WMI and IVH (p > 0.050). Also, cerebellar hemorrhage on MRI was noted in 31/181 (17.0%) neonates. Strikingly, a higher proportion of neonates with LOS developed cerebellar hemorrhage compared to those with EOS (24.5% vs. 18.8%) and those without sepsis (28.1% vs. 5.4%; p <0.001 [Table 1].
Adjusting for birth weight, gestational age, severe sepsis, and NEC, a significant association was seen between LOS and the risk of cerebellar hemorrhage (adjusted odds ratio = 4.6; 95%CI: 1.3–18.6). The risk of cerebellar hemorrhage increased after infection with K. pneumonia (58.1%) and E. faecalis (22.6%) [Table 2].
Neurodevelopmental outcomes were evaluated for 168 infants using the BSID-III scoring by 36 months CA (median: 34 months, IQR: 33–36 months). Between discharge and 34-month assessment, two infants died, eight were lost during follow-up, while three had a severe impairment and were unable to complete the test. The univariate analysis revealed a significant association between LOS and lower Bayley-III composite scores for all three domains: motor (p < 0.001), cognitive (p = 0.027), and language (p = 0.044). Also, LOS was associated with a higher risk of CP (28.0%, p = 0.003) [Table 3]. Figure 1 compares the groups based on their BSID-III scale composite scores. However, the multivariate regression analysis (adjusting for gestational age, birth weight, cerebellar hemorrhage, and white matter injury) revealed that the LOS group was had significantly lower motor composite scores (β coefficient = –9.5, 95% CI: –16.4 to –2.7), but not with cognitive and language composite scores (p > 0.050) [Table 4].
Discussion
This study is a unique analysis of neonatal LOS and its influence on brain injury and the later neurodevelopmental outcomes by 3 years of age. The neonates with LOS had comparatively lower gestational age, birth weight, length, and head circumference. A history of neonatal LOS made later cerebellar hemorrhage highly likely. However, the risk for of WMI and IVH was similar for all neonates in this study irrespective of their sepsis history. Our results also reaffirm the concept that LOS negatively affects neurodevelopmental outcomes, particularly motor, as evident from the LOS group’s lower BSID-III motor composite scores and their susceptibility for CP.
Sepsis may lead to BPD, requiring steroid therapy to facilitate extubation in these patients and mitigate other complications of a preterm birth like adrenal insufficiency and arterial hypotension.29–31 A series of studies evaluated the incidence of both EOS (1.5 per 1000 live births) and LOS (11.63 per 1000 live births) in the Arabian Gulf region, highlighting the disease burden because of LOS contracted in the NICU.32,33 Furthermore, our results establish a significant association of severe sepsis and complications like CLD, PDA, NEC, hypotension, and ROP through univariate logistic regression analysis.
These complications adversely affect the developing brain, as demonstrated by Stoll et al (2002),15 who found that infants with sepsis, sepsis with NEC, or meningitis were significantly predisposed to poorer neurodevelopmental outcomes evident by 18–22 months.11,13,14 Likewise, another study described neonatal sepsis with NEC to be associated with increased neurodevelopmental impairment in low birth-weight survivors manifesting as CP.16 Several human and animal studies have verified the presence of bacterial exotoxins-induced cytokine-release from microglia and astrocytes, resulting in a systemic inflammatory response syndrome, which further increases the blood-brain barrier permeability causing neuronal damage and apoptosis.34–37 The consequent arterial hypotension and the lability of blood pressure during sepsis, coupled with coagulopathy, lead to cerebral ischemia-reperfusion injury impairing neuronal oxygenation and cerebral autoregulation.11 These events, supplemented by the biological prematurity of the nervous tissue render it vulnerable to injury, presenting as neurocognitive or neuromotor developmental deficits.38,39
Some reports consider CONS bacteria as the commonest organisms causing LOS.12 However, in our study, LOS was caused mainly by two gram-negative organisms, K. pneumonia and E. coli. Mortality and morbidity caused by gram-negative bacteria are known to be significantly higher.12 Gram-negative bacteria’s camouflaging mechanism helps it evade early detection by the host immune system. Furthermore, in neonatal sepsis, gram-negative bacteria strongly attack the platelets, triggering thrombocytopenia and coagulopathy.40 Hence, the higher risk of cerebellar hemorrhage with gram-negative infection. Barring the effect of location and extent of hemorrhage, 43%–75% of infants who develop cerebellar hemorrhage experience serious delays in language, motor, cognitive, and behavioral development.41 Our study also revealed exceptionally low gestational age to be an additional risk factor for cerebellar hemorrhage.
This study also corroborates the importance of early quantitative MRI in preterm neonates, especially in cases with neonatal sepsis. Cayam-Rand et al,42 recommended the use of MRI coupled with clinical factors to detect and localize WMI and predict developmental outcomes by preschool age.42 Although we did not localize WMI, a significant association of cerebellar hemorrhage on MRI with neurodevelopmental outcomes, particularly motor, can be deduced from our results. These results also highlight the importance of using advanced serial MRI in the NICU to delineate the role of prematurity-induced cerebellar hemorrhage and topographically correlate with the developmental outcomes assessment.
We also identified a strong association between NICU-contracted LOS and later neurodevelopmental deficits. The processes of cellular migration, proliferation, and arborization essential for the fetus’ neurological development occur during the third trimester as a highly dynamic progression. Hence, any insult to the developing brain, be it preterm birth, intrauterine infection, or the systemic inflammatory response to LOS, will have neurological consequences, as shown in this study.
This study has some limitations. The retrospective nature of the study may have caused a selection bias in data collection; however, the authors managed to retrieve almost all the relevant information. Second, the study sample was small and single-centric, limiting the generalizability of the results. However, our results showed enough statistical significance to prove the association of LOS with cerebellar hemorrhage in premature infants. Future research should cover a larger proportion of the population across the Arabian Gulf region to identify any confounding factors influencing neurodevelopmental outcomes.
Conclusion
LOS in a premature newborn is associated with higher probability of cerebellar hemorrhage and other neonatal comorbidities, and poorer neurodevelopmental outcomes. Infection by gram-negative bacteria (particularly K. pneumoniae and E. coli) further deteriorates the neurological prognosis. This study provides a greater understanding of the risk factors linked with poor neurodevelopmental outcomes that can either be averted or managed using preventive and therapeutic strategies.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. de Haan TR, Beckers L, de Jonge RC, Spanjaard L, van Toledo L, Pajkrt D, et al. Neonatal gram negative and Candida sepsis survival and neurodevelopmental outcome at the corrected age of 24 months. PLoS One 2013;8(3):e59214.
- 2. Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014 Aug;14(8):751-762.
- 3. Pek JH, Yap BJ, Gan MY, Seethor ST, Greenberg R, Hornik CP, et al. Neurocognitive impairment after neonatal sepsis: protocol for a systematic review and meta-analysis. BMJ Open 2020 Jun;10(6):e038816.
- 4. Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS; Canadian Neonatal Network. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks’ gestation. Am J Perinatol 2015 Jun;32(7):675-682.
- 5. Downey LC, Smith PB, Benjamin DK Jr. Risk factors and prevention of late-onset sepsis in premature infants. Early Hum Dev 2010 Jul;86(Suppl 1):7-12.
- 6. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed 2015 May;100(3):F257-F263.
- 7. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al; National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004 Nov;292(19):2357-2365.
- 8. Joseph CJ, Lian WB, Yeo CL. Nosocomial infections (late-onset sepsis) in the neonatal intensive care unit (NICU). Proceed Singapore Healthcare 2012;21(4):238-244.
- 9. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet 2017 Oct;390(10104):1770-1780.
- 10. Schneider J, Miller SP. Preterm brain injury: white matter injury. Handb Clin Neurol 2019;162:155-172.
- 11. Zonnenberg IA, van Dijk-Lokkart EM, van den Dungen FA, Vermeulen RJ, van Weissenbruch MM. Neurodevelopmental outcome at 2 years of age in preterm infants with late-onset sepsis. Eur J Pediatr 2019 May;178(5):673-680.
- 12. Greenberg RG, Kandefer S, Do BT, Smith PB, Stoll BJ, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Late-onset Sepsis in extremely premature infants: 2000-2011. Pediatr Infect Dis J 2017 Aug;36(8):774-779.
- 13. Hemels MA, Nijman J, Leemans A, van Kooij BJ, van den Hoogen A, Benders MJ, et al. Cerebral white matter and neurodevelopment of preterm infants after coagulase-negative staphylococcal sepsis. Pediatric Critical Care Medicine 2012;13(6):678-684.
- 14. Xiong T, Gonzalez F, Mu DZ. An overview of risk factors for poor neurodevelopmental outcome associated with prematurity. World J Pediatr 2012 Nov;8(4):293-300.
- 15. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD neonatal research network. Pediatrics 2002 Aug;110(2 Pt 1):285-291.
- 16. Pawar SJ, Oleti T, Bharathi S, Tipparaju S, Mustafa E. Growth and neurodevelopmental outcome in preterm LBW infants with sepsis in India: a prospective cohort. Int J Pediatr 2018 Feb;2018:5735632.
- 17. Hinojosa-Rodríguez M, Harmony T, Carrillo-Prado C, Van Horn JD, Irimia A, Torgerson C, et al. Clinical neuroimaging in the preterm infant: diagnosis and prognosis. Neuroimage Clin 2017 Aug;16:355-368.
- 18. Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005 Nov;147(5):609-616.
- 19. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 2003 Aug;143(2):171-179.
- 20. Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008 Aug;153(2):170-175, 175.e1.
- 21. Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics 2008 Aug;122(2):299-305.
- 22. Lim WH, Lien R, Huang YC, Chiang MC, Fu RH, Chu SM, et al. Prevalence and pathogen distribution of neonatal sepsis among very-low-birth-weight infants. Pediatr Neonatol 2012 Aug;53(4):228-234.
- 23. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978 Jan;187(1):1-7.
- 24. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988 Oct;82(4):527-532.
- 25. An international classification of retinopathy of prematurity. The committee for the classification of retinopathy of prematurity. Br J Ophthalmol 1984;68(10):690-697.
- 26. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978 Apr;92(4):529-534.
- 27. Michalec D. Bayley scales of infant development: third edition. In: Goldstein S, Naglieri JA, editors. Encyclopedia of child behavior and development. Springer, Boston; 2011.
- 28. Synnes A, Luu TM, Moddemann D, Church P, Lee D, Vincer M, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Childhood Fetal Neonat Ed 2017;102(3):F235-F234.
- 29. Tam EW, Chau V, Ferriero DM, Barkovich AJ, Poskitt KJ, Studholme C, et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci Translat Med 2011;3(105):105ra.
- 30. Chang CH, Chang FM, Yu CH, Ko HC, Chen HY. Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med Biol 2000 Jul;26(6):981-988.
- 31. Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 2009 Sep;24(9):1085-1104.
- 32. Hammoud MS, Al-Taiar A, Al-Abdi SY, Bozaid H, Khan A, AlMuhairi LM, et al. Culture-proven early-onset neonatal sepsis in Arab states in the gulf region: two-year prospective study. Int J Infect Dis 2017 Feb;55:11-15.
- 33. Hammoud MS, Al-Taiar A, Al-Abdi SY, Bozaid H, Khan A, AlMuhairi LM, et al. Late-onset neonatal sepsis in Arab states in the gulf region: two-year prospective study. Int J Infect Dis 2017 Feb;55:125-130.
- 34. Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol 2009 Sep;24(9):1119-1126.
- 35. Nwafor DC, Brichacek AL, Mohammad AS, Griffith J, Lucke-Wold BP, Benkovic SA, et al. Targeting the blood-brain barrier to prevent sepsis-associated cognitive impairment. J Cent Nerv Syst Dis 2019 Apr;11:1179573519840652.
- 36. McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol Res Int 2012;2012:561494.
- 37. Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al; Trial of Indomethacin Prophylaxis in Preterms Investigators. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 2009 Jan;123(1):313-318.
- 38. Kurtom W, Jain D, Quan M, Vanbuskirk S, Bancalari E, Claure N. The impact of late onset arterial hypotension on respiratory outcome in extremely premature infants. Neonatology 2019;115(2):164-168.
- 39. Silveira RC, Procianoy RS. Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J Pediatr 2003 Nov;143(5):625-629.
- 40. Villamor-Martinez E, Fumagalli M, Alomar YI, Passera S, Cavallaro G, Mosca F, et al. Cerebellar hemorrhage in preterm infants: a meta-analysis on risk factors and neurodevelopmental outcome. Front Physiol 2019 Jun;10:800.
- 41. Hortensius LM, Dijkshoorn AB, Ecury-Goossen GM, Steggerda SJ, Hoebeek FE, Benders MJ, et al. Neurodevelopmental consequences of preterm isolated cerebellar hemorrhage: a systematic review. Pediatrics 2018 Nov;142(5):e20180609.
- 42. Cayam-Rand D, Guo T, Grunau RE, Benavente-Fernández I, Synnes A, Chau V, et al. Predicting developmental outcomes in preterm infants: a simple white matter injury imaging rule. Neurology 2019 Sep;93(13):e1231-e1240.