Trigger digit (TD) is one of the most frequent pathologies of the hand. In the non-diabetic population, the incidence rate is 2.2% throughout life for those over 30 years old, and the incidence is four times higher in the diabetic population.1,2 Three different surgical techniques have been described for releasing the first annular (A1) pulley in TD: classic open surgery (COS),3,4 blind percutaneous release (BPR),5,6 and ultrasound-guided A1 pulley release (USGAR).7–9
COS has been related to dissatisfaction rates of up to 26%10 and complications including stiffness,11 complex regional pain syndrome,12 and persistent local pain.13 BPR, despite excellent short-term results, still raises some concerns in terms of achieving a complete release,14 and due to the risk of damaging collateral structures.15 Furthermore, some authors have suggested restricting BPR to the third and fourth digits.16
In the last 10 years, ultrasound-guided procedures for treating TD have shown excellent results in every digit without major complications.8,17 Recent randomized control trials showed significantly better results with USGAR techniques than COS18 and BPR19 in terms of early recovery and release rate, respectively. However, it remains unclear the optimal surgical device (needle9 or hook knife8,17), the positioning of the instrument (extrasheath17 or intrasheath8), or the direction of the cut (anterograde9 or retrograde8,17).
The authors of a cadaveric study8 described a safe area palmar to the tendon sheath for releasing A1 pulley with a new intrasheath percutaneous ultrasound-guided technique (intrasheath-USGAR) using a hook knife. The same authors, in a later prospective clinical study, showed the efficacy and safety of their technique.20
The objectives of this pilot study were to compare clinically intrasheath-USGAR versus COS, and to evaluate the feasibility of a future clinical trial in terms of safety, efficacy, sample size, and procedures for patients with TD.
Methods
This randomized, parallel-group, controlled, external pilot study was performed in Madrid, Spain, in an ambulatory setting, between April and October 2010, with a follow-up of three months. Institutional review board approval and written informed consent were obtained.
We used Froimson’s classification21 ranging from grade I to IV: ‘pain without catching’ (grade I), ‘catching solved with active flexion/extension’ (grade II), ‘catching that needs passive flexion/extension’ (grade III), and ‘fix contracture’ (grade IV). Inclusion criteria were patients with signs of primary grade III TD for at least two months. Exclusion criteria were age < 18, previous pathology of the upper limb, malformations, and secondary TD. For ambulatory surgery, we excluded patients > 84 years old, allergies to local anesthesia or latex, smoking more than 20 cigarettes per day, heavy alcohol intake (> 60 g per day), oral anticoagulation, rheumatic disease, fibromyalgia, active psychiatric disease, blood pressure > 155/95 mmHg, BMI ≥ 40, pregnancy, cardiovascular or noncontrolled renal, hepatic, or hematologic disease, and hospital admission six months before surgery.20 The second author confirmed the inclusion criteria and performed all the procedures with a portable ultrasound scanner (LOGIQ Book XP Pro, 5-11 MHz 8L, GE Healthcare, Madrid, Spain). Outcome assessors were blinded by covering the patient’s digit. We performed concealed allocation (1:1), by an independent blocked computer-generated list, assigning patients to one of the two study groups: intrasheath-USGAR or COS.
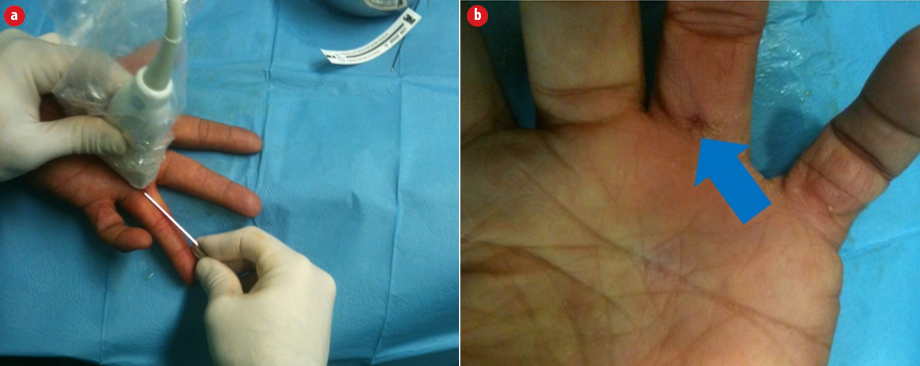
Figure 1: Surgical details for intrasheath-USGAR. (a) Distal volar approach at the proximal phalangeal crease. (b) Skin incision right after surgical release.
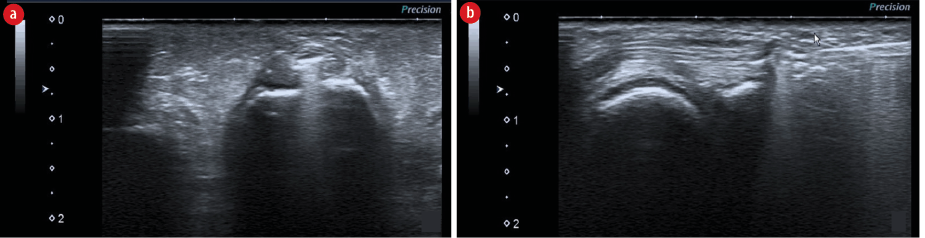
Figure 2: Ultrasound images of the intrasheath-USGAR procedure. (a) Introduction of the hook knife inside the tendon sheath with its cutting edge sideways (transverse position). (b) A1 pulley release with the edge toward the palm (longitudinal position).
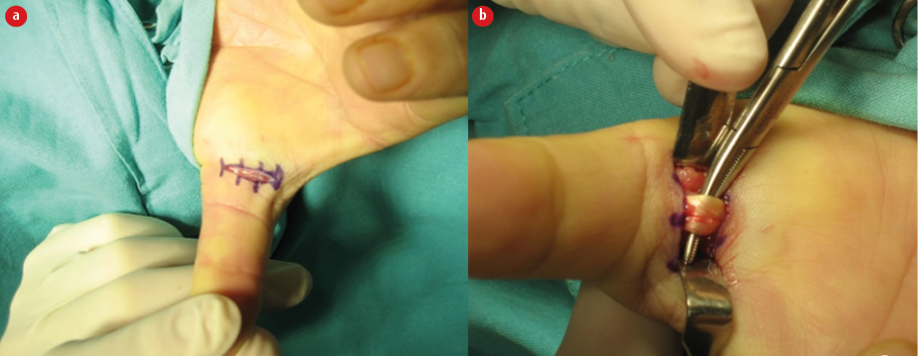
Figure 3: Surgical details for COS in a trigger thumb. (a) Location and incision size. (b) Flexor tendon after A1 pulley release.
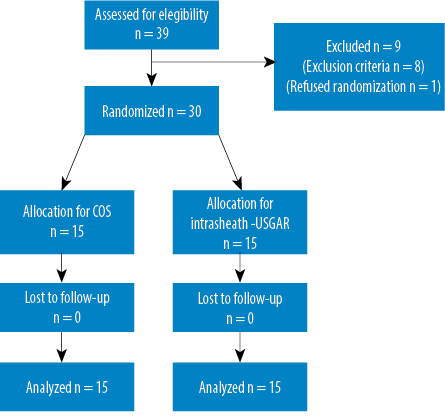
Figure 4: Patient flow diagram showing participant progress.
Table 1: Procedural issues objectives.
|
Recruitment rates |
70% of eligible patients included.
≤ 5% of eligible patients refused randomization.
10 patients included in the study per month. |
30 of 39 eligible patients included (76.9%).
1 (3.3%) refused randomization.
20 patients included per month. |
|
Blinding |
> 90% of the randomized patients. |
100% were operated blindly. |
|
Compliance |
> 90% of cases completed all interviews. |
Compliance was 98.5%. |
|
Completion |
More than > 90% completed the last interview. |
Completion was 100%. |
Table 2: Clinical variables.
|
Days for stopping oral analgesics |
2.4 ± 0.9 |
10.0 ± 3.8 |
|
Days for complete digit extension |
1.8 ± 1.0 |
7.2 ± 3.3 |
|
Days for complete finger digit |
2.8 ± 0.9 |
8.0 ± 3.5 |
Values are represented as mean±SEM. Intrasheath-USGAR: intrasheath ultrasound guided A1 pulley release; COS: classic open surgery.
*: statistically significant, p < 0.050.
.png)
Figure 5: (a) QuickDASH and (b) grip strength after intrasheath-USGAR (blue) or COS (black). Prior to surgery (Presurg) and postoperatively (three, six, and 12 weeks). The grip rate is calculated as a percentage of the individual’s normal grip distinguishing the dominant or non-dominant hand. Strength of dominant uninjured side - 10% = calculated normal strength of the injured non-dominant side or strength of non-dominant uninjured side+10% = calculated normal strength of the injured dominant side. Variables expressed as mean±SEM. *p < 0.050; **p > 0.050.
The USGAR followed the technique described by Rojo-Manaute et al,20 which consisted of introducing a sonographically guided 16-gauge Abbocath (Abbott Laboratories, North Chicago, IL) 1 cm distal to the volar metacarpophalangeal crease of the thumb and the volar proximal phalangeal crease of the rest of the fingers aiming for a point of entry in the volar tendon sheath located 3 mm distal from the base-shaft junction of the proximal phalanx. We then placed our cutting tool [Figure 1] (a retrograde knife, 5151-A; Orthomed SA, St Jeannet, France; or 010600 Acufex hook knife, 3.0 mm; Smith & Nephew, Memphis, TN) in an intrasheath position [Figure 2] and pushed it to the proximal cutting point to release the A1 pulley by turning the edge toward the palm and pulling it to the point of entry.
COS was performed under local anesthesia without ischemia by performing a 1 cm incision at the metacarpophalangeal crease and releasing the A1 pulley under direct visualization, after dissecting the skin and subcutaneous tissue [Figure 3].22
Success was determined if all feasibility objectives for our pilot study were matched. Primary objectives included safety (absence of neurovascular morbidity) and efficacy (no TD recurrence three months after surgery). Secondary objectives (procedural issues) are defined in Table 1. Sample size calculation was done using Epidat 3.1 based on the mean ± standard deviation values for QuickDASH (primary outcome measure for a future randomized clinical trial) at six weeks. Ten percent more patients were added to the sample for taking into account any possible losses to follow-up.23
The clinical variables included preoperatively were symptoms duration, QuickDASH, active worker or retired, and previous conservative treatments. Postoperatively at one, three, and six weeks, and three months we measured QuickDASH, grip strength (JAMAR, Hydraulic Hand Dynamometer. Bolingbrook, IL, USA) and two points of discrimination. We also recorded the recovery time (in days) until they stopped using pain killers and the time taken to have full digit range of motion and resume their daily activities (including work). Any complications were also reported.
Mean and standard error of the mean (SEM) were recorded for QuickDASH, grip strength, and mean (SEM) and range for the clinical variables. We used SPSS Statistics for Windows, version 15.0 (SPSS Inc., Chicago, Ill., USA) for the analysis. Student’s t-test and chi-square (statistically significant at p < 0.050) with no power calculation was performed.
Results
Thirty of 39 eligible patients were randomized to either the intrasheath-USGAR or the COS group [Figure 4]. Patient’s background data showed no significant differences in average age (59.6 (range: 36–77) vs. 58.1 (range: 42–74) years), previous symptom duration (11.8 (range: 4–30) vs. 12.2 (range: 2–35) months), active workers (16 (53.3%) vs. 13 (40.3%)), or sex (7 females (46.7%) vs. 6 males (40%)).
There was no neurovascular morbidity or recurrence in both groups. The results for our feasibility objectives are detailed in Table 1. We calculated that a randomized controlled trial would require a sample size of 76 patients (power: 80%; confidence level: 95%). Ten percent more patients were added to the sample to consider any possible losses, giving a total of 84.
The average values for QuickDASH was significantly lower for intrasheath-USGAR (8.7±5.6) than for COS (21.6±6.7) at six weeks. QuickDASH and grip strength results are shown in Figure 5. There were no differences between groups in our clinical variables except for the number of days taken to return to normal daily activities, which favored the USGAR group [Table 2]. In the COS group, we had a case with local moderate pain that persisted until the third month. No major complications were reported in either group.
Discussion
The goal when treating TD is to fix the mechanical mismatch between the A1 pulley and the flexor tendon. Surgically, COS3,4 and BPR6,19 have shown a similar success rate (> 90%). However, despite these promising results, the difficulty of obtaining a complete release in BPR,14 the risk of injuring collateral structures,15 and major complications associated with COS4,12 have raised some doubts about the two traditional surgical options.
The surgical success rate is defined for TD as a postoperative absence of triggering. Different authors4,8,9,17 have described various USGAR techniques for treating TD, with excellent success rates (91%–100%) in every digit without major complications. Unfortunately, there are still some concerns about its generalization, efficacy, and safety due to multiple factors: 1) the relative position of the cutting device respective to the synovial sheath; 2) the direction of the cut; and 3) cutting device. Rojo et al,8 described first in cadavers and then clinically,20 an intrasheath-USGAR with excellent clinical results in terms of safety and efficacy, generalizable to every digit without major complications.
The purpose of pilot studies is to assess the feasibility in terms of safety, efficacy, procedural issues, and sample size calculation.24,25 Our external pilot study showed that our USGAR release for TD was safe and effective in both groups and that we matched our procedural objectives for recruitment, blinding, compliance, and completion rates [Table 1].24 The concealment of the operated digit supposed a saturation of our auxiliary staff, so we asked patients to cover the digit with an adhesive dressing by themselves before the interview with the data collector. We used the QuickDASH scale as our primary variable given the international validity shown in hand disorders. Our study had a follow-up period of three months, which may seem short, however, a previous study observed that the USGAR technique showed an almost normal average QuickDASH score by the sixth postoperative week and normal scores by the sixth month.20 Moreover, the authors of another study26 did not observe any significant differences between their open and minimally invasive groups after the eighth postoperative week. Thus, by setting the duration of our pilot study to three months we attempted to detect differences between both surgical techniques until the third month, since we believed that both techniques would not have significant differences after this time based on the previous literature. Our preliminary clinical results showed that intrasheath-USGAR had a shorter recovery time for restarting normal daily activities.
Our limitations were related to the procedure and the scarce existing literature about pilot studies.25 First, a single surgeon performed all the operations with the intention of standardizing the procedure and avoiding interindividual differences and there is a learning curve to the USGAR technique. Our first clinical patient took 35 minutes to achieve a release. At present, a release takes three to four minutes. Second, the nature of the procedure made it impossible to blind the type of surgery made to each patient participating in the study. This issue has been addressed in the CONSORT 2010 guidelines27 which points out that “in certain trials, especially surgical trials, blinding of participants and surgeons is often difficult or impossible”. Third, we included all the parameters found in the literature for this kind of pilot study (safety, efficacy, recruitment rates, blinding, compliance, completion rates, preliminary results, and sample size calculations).25,28 However, there is no a clear guideline for establishing the success thresholds for each of these variables. Thus, what we did was set the thresholds based on the more accepted methodology at the moment.29 According to the pilot study by Choi et al,30 we set the success in our recruitment rate in > 70%. Similarly, we fixed the sample size calculation in 30 patients based on the recommendations for pilot studies given by Lancaster et al,24 who recommend taking at least 30 patients, and Arnold et al,28 who suggested a median number of 52 (average 59.6, range 20–120).
Conclusion
A randomized clinical trial comparing COS versus intrasheath-USGAR is feasible in terms of potential safety, efficacy, and sample size calculation. The protocol of data gathering should be modified in the patients’ concealment item. The posterior clinical trial will confirm or refuse the generalization of the new intrasheath-USGAR technique in patients with symptomatic TD.
Disclosure
The authors declared no conflicts of interest. This study was funded in part by a grant from MAPFRE (volume 19, number 4, October/December, 2008).
Acknowledgements
The authors would like to thank Drs. Emilio Rodríguez Bilbao and Eugenio Cerezo López for their essential support and Daragh Malone and Cristina Fernández Buergo for helping with the translation.
references
- Saldana MJ. Trigger digits: diagnosis and treatment. J Am Acad Orthop Surg 2001 Jul-Aug;9(4):246-252.
- 2. Stahl S, Kanter Y, Karnielli E. Outcome of trigger finger treatment in diabetes. J Diabetes Complications 1997 Sep-Oct;11(5):287-290.
- 3. Gilberts EC, Beekman WH, Stevens HJ, Wereldsma JC. Prospective randomized trial of open versus percutaneous surgery for trigger digits. J Hand Surg Am 2001 May;26(3):497-500.
- 4. Hansen RL, Søndergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am 2017 May;42(5):359-366.
- 5. Abe Y. Clinical results of a percutaneous technique for trigger digit release using a 25-gauge hypodermic needle with corticosteroid infiltration. J Plast Reconstr Aesthet Surg 2016 Feb;69(2):270-277.
- 6. Zyluk A, Jagielski G. Percutaneous A1 pulley release vs steroid injection for trigger digit: the results of a prospective, randomized trial. J Hand Surg Eur Vol 2011 Jan;36(1):53-56.
- 7. Jou IM, Chern TC. Sonographically assisted percutaneous release of the a1 pulley: a new surgical technique for treating trigger digit. J Hand Surg Br 2006 Apr;31(2):191-199.
- 8. Rojo-Manaute JM, Soto VL, De las Heras Sánchez-Heredero J, Del Valle Soto M, Del Cerro-Gutiérez M, Martín JV. Percutaneous intrasheath ultrasonographically guided first annular pulley release: anatomic study of a new technique. J Ultrasound Med 2010 Nov;29(11):1517-1529.
- 9. Rajeswaran G, Lee JC, Eckersley R, Katsarma E, Healy JC. Ultrasound-guided percutaneous release of the annular pulley in trigger digit. Eur Radiol 2009 Sep;19(9):2232-2237.
- 10. Thorpe AP. Results of surgery for trigger finger. J Hand Surg Br 1988 May;13(2):199-201.
- 11. Lim MH, Lim KK, Rasheed MZ, Narayanan S, Beng-Hoi Tan A. Outcome of open trigger digit release. J Hand Surg Eur Vol 2007 Aug;32(4):457-459.
- 12. Callegari L, Spanò E, Bini A, Valli F, Genovese E, Fugazzola C. Ultrasound-guided injection of a corticosteroid and hyaluronic acid: a potential new approach to the treatment of trigger finger. Drugs R D 2011;11(2):137-145.
- 13. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics 2013 Oct;36(10):e1290-e1294.
- 14. Hoang D, Lin AC, Essilfie A, Minneti M, Kuschner S, Carey J, et al. Evaluation of percutaneous first annular pulley release: efficacy and complications in a perfused cadaveric study. J Hand Surg Am 2016 Jul;41(7):e165-e173.
- 15. Habbu R, Putnam MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am 2012 Nov;37(11):2273-2277.
- 16. Eastwood DM, Gupta KJ, Johnson DP. Percutaneous release of the trigger finger: an office procedure. J Hand Surg Am 1992 Jan;17(1):114-117.
- 17. Chern TC, Jou IM, Yen SH, Lai KA, Shao CJ. Cadaveric study of sonographically assisted percutaneous release of the A1 pulley. Plast Reconstr Surg 2005 Mar;115(3):811-822.
- 18. Nikolaou VS, Malahias MA, Kaseta MK, Sourlas I, Babis GC. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop 2017 Feb;8(2):163-169.
- 19. Lee SH, Choi YC, Kang HJ. Comparative study of ultrasonography-guided percutaneous A1 pulley release versus blinded percutaneous A1 pulley release. J Orthop Surg (Hong Kong) 2018 May-Aug;26(2):2309499018772368.
- 20. Rojo-Manaute JM, Rodríguez-Maruri G, Capa-Grasa A, Chana-Rodríguez F, Soto MdelV, Martín JV. Sonographically guided intrasheath percutaneous release of the first annular pulley for trigger digits, part 1: clinical efficacy and safety. J Ultrasound Med 2012 Mar;31(3):417-424.
- 21. Froimson AI. Tenosynovitis and tennis elbow. In: Green DP, editor. Green’s operative hand surgery. 3rd ed. New York: Churchill Livingstone; 1993. p. 1995-1998.
- 22. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am 2006 Jan;31(1):135-146.
- 23. Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care 2002 Aug;6(4):335-341.
- 24. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004 May;10(2):307-312.
- 25. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al; PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016 Oct;355:i5239.
- 26. Sato ES, Gomes Dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology (Oxford) 2012 Jan;51(1):93-99.
- 27. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al; Consolidated Standards of Reporting Trials Group. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010 Aug;63(8):e1-e37.
- 28. Arnold DM, Burns KE, Adhikari NK, Kho ME, Meade MO, Cook DJ; McMaster Critical Care Interest Group. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med 2009 Jan;37(1)(Suppl):S69-S74.
- 29. Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010 Jan;10:1.
- 30. Choi PT, Beattie WS, Bryson GL, Paul JE, Yang H. Effects of neuraxial blockade may be difficult to study using large randomized controlled trials: the perioperative epidural trial (POET) pilot study. PLoS One 2009;4(2):e4644.