Type 1 diabetes mellitus (T1DM) usually occurs due to insulinopenia either secondary to the autoimmune process (as in most cases) or idiopathic.1–3 Diabetic ketoacidosis (DKA) is a leading cause of hospitalization and mortality in children with T1DM, which makes studying DKA, its frequency, and the characteristics of hospital admissions important.4,5 In Saudi Arabia, the prevalence of T1DM in children and adolescents was reported as 109.5 per 100 000.6 The frequency of DKA among T1DM children in Saudi Arabia is double the world’s average.7 In many cases, DKA can be the first presentation of T1DM in newly diagnosed patients.
However, it is usually associated with a precipitating factor in patients known to have T1DM and on insulin treatment. This could be due to infections or other illnesses, problems with insulinization in relation to technical or psychosocial issues, or due to other intercurrent events, such as surgery.
Many factors might influence morbidity and mortality in DKA. The severity of the DKA episode at presentation can potentially affect the outcome. Other parameters, such as mental status, anion gap, and serum osmolality, should be considered during severity assessment.8 Also, the length of hospital stay (LOS) is another important parameter that reflects on the morbidity of DKA. In general, the LOS for patients with diabetes depends on the severity, underlying cause, and clinical course during hospital admission.9
Studying the frequency and risk factors of DKA hospital admissions can reshape preventive strategies, including educational programs for high risk patients and potentially improve the quality of care provided to these patients by conducting further follow-up studies. A previous study in our old hospital had looked at the clinical and biochemical characteristics of DKA admissions in the pediatric department (1995–2008).10 Our study was conducted at the more recently established King Abdullah Specialized Children’s Hospital (KASCH), assessing the progress in patients’ care and clinical outcome of DKA. The advanced settings in KASCH included receiving care in specialized wards with trained nurses as well as easy access to high dependency and pediatric intensive care services, doubling the numbers of serving staff including physicians and diabetes educators with regular attendance of dieticians in diabetes clinics, conducting group educational activities and sessions on special events (such as before Ramadan) regularly, and the nearby presence of all collaborating specialties, such as ophthalmology and psychology/psychiatry services, in the new tertiary settings allowing easy review and referrals of these patients. Additionally, the center also runs adult diabetes clinics to facilitate proper transitional care and to promote establishing a diabetes center in the near future.
In this study, we aimed to assess the characteristics of hospital admissions with DKA in newly diagnosed and known patients with T1DM who received care in these advanced settings to facilitate further improvement and adjustment of the service.
Methods
This is a retrospective chart review of all children admitted with DKA. Data were extracted from electronic records of children ≤ 14 years old who had the biochemical criteria of DKA on admission (determined based on the first serum bicarbonate (HCO3)and pH level and categorized as mild < 7.3, moderate < 7.2, and severe < 7.1, respectively).11 Characteristics of hospital admissions were assessed in newly diagnosed and known patients with T1DM. These included DKA frequency, precipitating causes of DKA such as infections or missing insulin, severity, glycated hemoglobin (HbA1C) level before DKA episode, duration of DKA episodes in hours, and the LOS.
The study was conducted at KASCH, the only specialized children’s hospital in Saudi Arabia. It opened in early 2015 to provide tertiary care for children 0–14 years old. The endocrinology department provides inpatient and outpatient consultations, diagnostic evaluation, and management of children and adolescents with diabetes, mainly T1DM. The department also provides inpatient and outpatient care for diabetic children, including intensive education utilizing the latest technology for children with diabetes.
We targeted all DKA patients admitted between March 2015 and December 2017 to the pediatric wards of KASCH. We included those who fulfilled the biochemical criteria of DKA (pH < 7.30, HCO3 < 15 mmol/L, glucose > 11.2 mmol/L (200 mg/dL) and detection of ketone bodies in urine or blood.11 Newly diagnosed patients with T1DM were identified based on the criteria of the American Diabetes Association, which consist of a random plasma glucose of 11.2 mmol/L (200 mg/dL) or more with hyperglycemic symptoms or crisis.12 However, in known patients of T1DM with already established diagnoses, we reviewed their follow-up records for further information.
Patients labeled with infection as a precipitating cause of DKA had to fulfill the laboratory criteria and symptomatology of infections and not be determined just by the presence of leukocytosis. Social crisis was identified in the patient’s file with the support of the social services department. Occasionally, these were also reported and documented by diabetes educators or other members of the team, especially during annual reviews when overall inquiries were conducted about patients’ surroundings. Exclusion criteria consisted non-T1DM patients, patients with another diagnosis that affects ketone production (such as concomitant metabolic diseases), and patients with incomplete biochemical criteria of DKA (possibly DK but not DKA, i.e., without acidosis).
The sample size of this study was calculated using the formula.
|
N = [z [of 1- α]2 p (1-p)] |
|
d2 |
Where p is the previously reported prevalence of DKA (55.3%) among patients with T1DM in Saudi Arabia.13 Therefore, with a margin of error of 5% and a confidence level of 95%, a recommended collective sample size of 96, which was rounded to 100, was deemed necessary to detect a significant difference. Data cleaning, management, and analyses were performed using SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Frequencies and percentages were presented for categorical variables such as gender, place of admission, and DKA severity. Means and standard deviations were calculated for numerical variables, such as HbA1C during DKA episode, duration of DKA recovery, and LOS. For inferential statistics, the chi-squared test was used to test the association between categorical variables, while the t-test and analysis of variance were used to test the association between categorical and numerical variables. A test was considered significant if the p-value was < 0.050.
Results
On average, we treated 562 children with T1DM patients annually in our institute during the study period, of which 141/562 (25.1%) were newly diagnosed versus 421/562 (74.9%) known patients with T1DM [Figure 1]. The frequency of DKA admissions among all patients with T1DM was 26.0% (n = 146/562), of which 29.8% (n = 42/141) were newly diagnosed and 24.7% (n = 104/421) were known patients [Figure 1]. DKA admissions constituted 146/311 (46.9%) of all inpatient admissions of children with T1DM during the study period. The total number of patients with DKA was 116 (mean age 8.9±3.0 years), and they had 146 DKA episodes [81 (55.5%) in females versus 65 (44.5%) in males] [Table 1]. The majority of DKA patients were aged 10–14 years (p < 0.001), of which 74/116 (63.8%) were previously known to have T1DM [Table 1 and Figure 1]. Missing insulin was the main identifiable precipitating cause (p = 0.001) of DKA in known patients with T1DM [Figure 2]. DKA recurrent episodes (n = 30/146, 20.5%) occurred in 15/116 patients. Recurrence was more common in children ≥ 10 years old (p = 0.024). In 3/15 patients who had recurrence, each had DKA recur five times during the study period representing 50% of the DKA recurrent episodes in our cohort. The first of these patients was an 11-year-old boy with a social crisis who was missing insulin and had these repeated episodes over eight months with one to two months gap between the episodes. The other two patients were girls, 12 and 16 years old, who were uncontrolled in their diabetes management and had DKA because of either infection or missing insulin doses for different reasons, including adolescents’ behavior and a fear of weight gaining with regular insulin treatment. When comparing children who had single and recurrent episodes of DKA, the latter group tends to have a shorter LOS (p < 0.001). Still, there was no difference in DKA severity or HbA1C level on admission between the two groups [Table 2]. The overall mean LOS was 2.6±2.0 days. Newly diagnosed patients had increased LOS duration and prolonged recovery from DKA [Table 2]. The severity of DKA was also associated with increased LOS, but not with the HbA1C level on admission [Table 3].
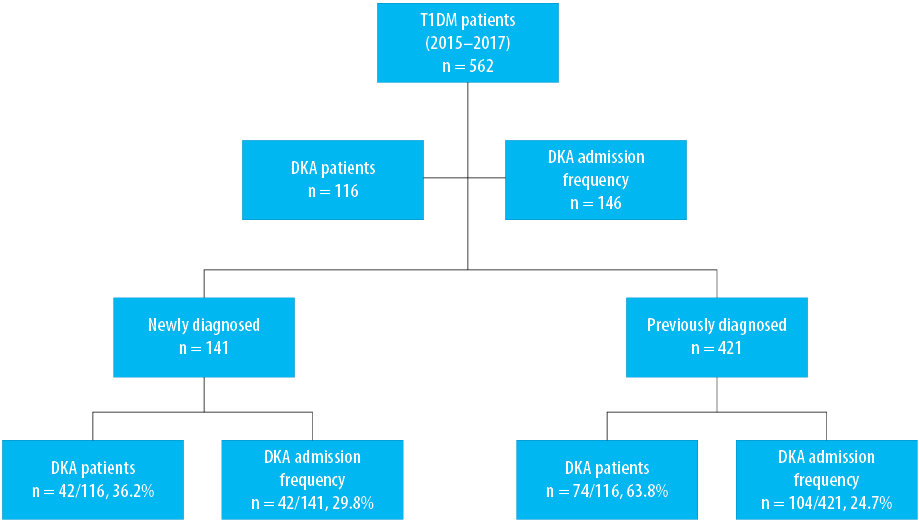
Figure 1: Frequency of diabetic ketoacidosis (DKA) in newly diagnosed and known patients with type 1 diabetes mellitus (T1DM).
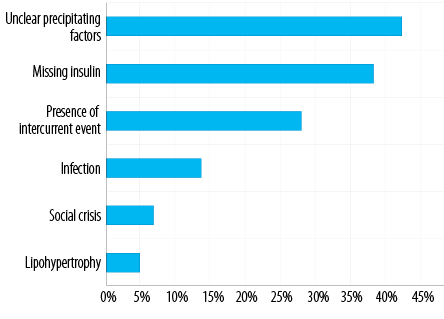
Figure 2: Precipitating factors of diabetic ketoacidosis in known patients with type 1 diabetes.
Table 1: Characteristics of admissions with diabetic ketoacidosis in newly diagnosed and known patients with type 1 diabetes mellitus (T1DM).
|
Age, years |
|
|
|
< 0.001 |
|
< 5 |
18 (12.3) |
12 (66.7) |
6 (33.30) |
|
|
5–10 |
66 (45.2) |
20 (30.3) |
46 (69.0) |
|
|
>10 |
62 (42.5) |
10 (16.1) |
52 (83.9) |
|
|
Gender |
|
|
|
0.066 |
|
Male |
65 (44.5) |
24 (36.9) |
41 (63.1) |
|
|
Female |
81 (55.5) |
18 (22.2) |
63 (77.8) |
|
|
Place of admission |
|
|
|
0.046 |
|
PICU |
52 (35.6) |
18 (34.6) |
34 (65.4) |
|
|
HDU |
20 (13.7) |
9 (45.0) |
11 (55.0) |
|
|
Ward |
74 (50.7) |
15 (20.3) |
59 (79.7) |
|
|
Severity |
|
|
|
1.000 |
|
Mild |
42 (28.8) |
12 (28.6) |
30 (71.4) |
|
|
Moderate |
98 (67.1) |
28 (28.6) |
70 (71.4) |
|
PICU: Pediatric intensive care unit; HDU: High dependency unit.
Table 2: Glycated hemoglobin, duration of ketoacidosis episode, and length of hospital stay in newly diagnosed and known patients with type 1 diabetes mellitus (T1DM).
|
HbA1C before DKA episode, % |
11.8 (10.7–13.2) |
11.1 (10.4–12.3) |
0.161 |
|
Duration of DKA episode, hours |
11 (10–20) |
9 (6–13) |
0.006 |
IQR: interquartile range; HbA1C: glycated hemoglobin; DKA: diabetic ketoacidosis ; LOS: length of stay.
Table 3: Association of the severity of diabetic ketoacidosis with length of hospital stay and glycated hemoglobin.
|
LOS, days |
Mild |
2.0 |
1.0 |
4.0 |
0.008 |
| |
Moderate |
2.0 |
1.0 |
3.0 |
|
| |
Severe |
4.5 |
4.0 |
10.0 |
|
|
HbA1C prior to DKA episode, % |
Mild |
11.0 |
10.4 |
13.0 |
0.434 |
| |
Moderate |
11.5 |
10.4 |
12.6 |
|
IQR: interquartile range; LOS: length of stay; HbA1C: glycated hemoglobin.
Discussion
DKA constitutes almost half of the admissions of patients with T1DM in our study. Approximately two-thirds of the patients admitted were known to have T1DM. This emphasizes the importance of investigating DKA admissions. Our institution has evolved into a specialized children’s center, and new protocols have been implemented which differ from the previous studies published from the same center.10 Missing insulin doses, intercurrent events, and infections were the common identifiable causes of DKA. Younger age was associated with recurrent DKA. In general, severity was associated with increased LOS, and younger patients have more recurrent episodes. Advanced services in a tertiary setting could improve patient identification and reduce the LOS in DKA management. The overall LOS was comparable to other international centers of similar settings.
DKA frequency among T1DM patients varies between different studies, populations, and countries.14 In the EURODIAB project, the frequency ranged from 26% to 67%.15 In Arab countries, the frequency ranged from 17% in Egypt to 100% in Algeria, Morocco, and Tunisia, as reported in a systematic review of 29 studies.16 In Saudi Arabia, the estimated frequency of DKA among children with T1DM ranged from 38% to 55.3%, but all of these studies were conducted outside Riyadh, the capital city of Saudi Arabia.17–20 Here, we detected a DKA frequency of 26.0% in newly and previously diagnosed T1DM patients, in agreement with previous findings. The leading cause of this considerable variation is not well known. However, many variables were inversely related to DKA frequency, such as expenditure on healthcare, gross domestic product, background incidence of T1DM, and latitude.14 A unique feature in Arab countries is that the higher the incidence and prevalence of T1DM, the higher the DKA frequency emphasizing the importance of intensifying awareness programs that increase public awareness.16 This could also be explained by a deficiency of resources in some clinical institutes where extra support is required. In this study, the transformation of the diabetes services into tertiary settings has led to improvement in the characteristics of DKA admission in our institute.10
Previous studies have shown that poor compliance with insulin treatment and infections are the main identifiable precipitating factors of DKA.21,22 Our study confirms these findings in known patients with T1DM, emphasizing the importance of rehearsing the sick-day rules periodically in outpatient clinics, especially in high-risk groups. During poor compliance to insulin, the presence of intercurrent events, such as surgery and acute social crisis, are additional contributing factors, though in a fewer number of cases. Missing insulin is probably more prevalent than reported in many studies, including ours, and it is very likely to be the cause of DKA in patients with unidentified precipitating factors despite thorough investigations during hospital admission. The majority of patients belonging to the ῾unidentified precipitating cause group were probably missing their insulin doses, although other factors might have a role that was not explored in our study. DKA could also be related to starting puberty, which is commonly associated with non-compliance with regular clinic visits and without adjusting their insulin doses. Inadequate insulin during a time of increased need puts these children at a higher risk of developing DKA.
As mentioned previously, infection is one of the leading causes of DKA in our study. Hamed et al,23 reported that infection accounted for 46.5% of DKA cases, with the most common sources of infection being urinary tract (31.2%) and respiratory tract infection (26.8%). Another study reported that infection was the leading cause of DKA in the intensive care unit and was associated with female gender, neurological symptoms at presentation, and lack of clearance of ketonuria.24
We compared our findings regarding age-gender distribution and risk factors to another performed in sub-tertiary settings in the same institute,10 and to other relevant studies. The mean age of patients with DKA has dropped to 8.9 (6–11) years from 11 (8–13) years.10 However, there are still higher rates of DKA admissions for children over 10. As reported by Randall et al,25 where younger age was significantly associated with recurrent DKA, our data showed that DKA recurrence was more common in children > 10 years old. There is also a drop in the percentage of newly diagnosed patients of DKA from 46% to 36.2% in our study, although the previous study data was of a longer duration.10 Missing insulin was the most common precipitating factor in both studies but was much lower in our study (39.0% vs. 79%).10 DKA duration had also dropped significantly in our tertiary center, to a median of 10 hours (6–16 hours) compared to 20 hours (12–28 hours),10 reflecting the value of advanced tertiary care settings. Of note, unlike the previous study at our center, we reported on the frequency of DKA in known as well as newly diagnosed patients with T1DM and on the recurrent episodes of DKA to inform further interventional strategies.
In previous reports, females had more recurrent DKA episodes than males, which was observed in our study.21,26 This could be related to the effect of insulin on weight gain, which is usually a major concern for female teenagers, making them skip insulin doses.21 Also, female patients with diabetes seem to have a higher risk of eating disorders, such as anorexia nervosa and bulimia.27 These psychological problems are associated with poor glycemic control and recurrence and can predispose patients to the rapid development of complications of diabetes, such as retinopathy.28 Our study showed a significant association of DKA in previously known patients with lipohypertrophy but not with psychosocial issues. Similarly, other studies have found a high prevalence of DKA in girls, in children less than five years old, and those who had lipodystrophy.29 Apart from the age factor, there were no other predictors of recurrence in our study.
One of the critical DKA clinical characteristics is the severity of the episode, as it guides different aspects of management. Education and awareness tend to reduce the severity of DKA as expected and was reported in a study conducted in the Northwest of Saudi Arabia, where the DKA rates decreased from 48% to 39% in four years.18 Patient’s education improves patient’s motivation and does not only reduce the severity and risk of recurrence of DKA episodes but also represents the cornerstone of preventing DKA in patients with T1DM.30 Proper education should focus on all elements of intensification of therapy, including multiple-component insulin regimen, a careful balance of food intake, activity, insulin dosage, daily self-monitoring of blood glucose, defined individualized target blood glucose levels, patient adjustments of food intake and insulin dosage, and use of insulin supplements according to a predetermined plan. The severity of DKA episodes has slightly improved in our study, with a mean biochemical pH level of 7.1±0.1 compared to 7.15±0.11 in Naeem et al.10 Overall, using more intensive insulin therapy in outpatient management in KASCH had positive effects on DKA events and patients glycemic control including milder episodes, shorter LOS, and lower HbA1c levels.
Usually, DKA patients are discharged within two days. However, some are reported to have high readmission rates within a month of discharge for various reasons.31 The LOS in our study was 2.6±2.0 days, similar to other studies of DKA in equivalent settings.31 The variability in LOS could depend on many factors. Hospitalization or LOS for patients with T1DM depends on the severity, underlying cause, and the clinical course during hospital admission.9 Patients with mild or moderate DKA tend to stay longer in the emergency room waiting area than patients with severe DKA.32 According to a study conducted in Ontario, Canada, the LOS for children with DKA has decreased from 4.5 to 3.2 days due to effective measures being taken.31 This included optimizing the medical care and effective use of ambulatory care in the form of a pediatric assessment unit that helped decrease the rate of admission of patients with T1DM to the
pediatric ward.31
This study helped assess some characteristics of DKA admissions and the risk factors for a Saudi population in Riyadh. However, since T1DM has multiple confounding factors that could affect clinical outcomes, including psychosocial wellbeing and family dynamics, a limitation of our study is that we did not explore the effects of these factors. In addition, this is a retrospective cohort study that should usually trigger further trial studies to implement changes in
clinical interventions.
Conclusion
Missing insulin is still a major cause of DKA in patients with known T1DM, even in advanced patient care settings. Adopting a system of intensive insulin therapy with adequate resources is essential to prevent or reduce the severity of DKA in high risk groups of adolescents, poorly controlled patients, and those with recurrent episodes. Patient education requires frequent contact between patient/parents and diabetes team to empower patients to contribute positively to their management.
Disclosure
The authors declare that the preliminary abstract and graphs were uploaded in a preprint platform called Authorea. It is an interactive website for scholars to upload their papers before peer-review. The DOI is added in the references.33 No funding was received for this study. This study was approved by King Abdullah International Medical Research Center in Riyadh, Saudi Arabia, with a reference number (RSS18/017/R).
Acknowledgements
The authors would like to thank the staff at KASCH, who was helpful in data collection. Also, we thank the Institutional Review Board and scientific committee at KAIMRC for reviewing, advising on the design of the study, and facilitating data collection, and would like to thank the statistical team at King Saud Bin Abdulaziz University for Health Sciences, Riyadh, for conducting the data analysis of this study.
references
- 1. Roglic G. WHO Global report on diabetes: a summary. Int J Noncommun Dis 2016 Apr;1(1):3-8.
- 2. Moini J. Type 1 diabetes. In: Epidemiology of diabetes. Elsevier; 2019. p. 75-90.
- 3. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010 Jan;33(Suppl 1):S62-S69.
- 4. Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectr 2002;15(1):28-36.
- 5. Vellanki P, Umpierrez GE. Increasing hospitalizations for DKA: a need for prevention programs. Diabetes Care 2018 Sep;41(9):1839-1841.
- 6. Al-Herbish AS, El-Mouzan MI, Al-Salloum AA, Al-Qurachi MM, Al-Omar AA. Prevalence of type 1 diabetes mellitus in Saudi Arabian children and adolescents. Saudi Med J 2008 Sep;29(9):1285-1288.
- 7. Robert AA, Al-Dawish A, Mujammami M, Dawish MA. Type 1 diabetes mellitus in Saudi Arabia: a soaring epidemic. Int J Pediatr 2018 May;2018:9408370.
- 8. Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician 2005 May;71(9):1705-1714.
- 9. Guisado-Vasco P, Cano-Megías M, Carrasco-de la Fuente M, Corres-González J, Matei AM, González-Albarrán O. Clinical features, mortality, hospital admission, and length of stay of a cohort of adult patients with diabetic ketoacidosis attending the emergency room of a tertiary hospital in Spain. Endocrinol Nutr 2015 Jun-Jul;62(6):277-284 .
- 10. Naeem MA, Al-Alem HA, Al-Dubayee MS, Al-Juraibah FN, Omair A, Al-Ruwaili AS, et al. Characteristics of pediatric diabetic ketoacidosis patients in Saudi Arabia. Saudi Med J 2015 Jan;36(1):20-25.
- 11. Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician 2013 Mar;87(5):337-346.
- 12. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetesd2021. Diabetes Care 2021;44(Suppl. 1):S152S33.
- 13. Al Shaikh A, Farahat F, Saeedi M, Bakar A, Al Gahtani A, Al-Zahrani N, et al. Incidence of diabetic ketoacidosis in newly diagnosed type 1 diabetes children in western Saudi Arabia: 11-year experience. J Pediatr Endocrinol Metab 2019 Aug;32(8):857-862.
- 14. Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 2012 Nov;55(11):2878-2894.
- 15. Lévy-Marchal C, Patterson CC, Green A; EURODIAB ACE Study Group. Europe and Diabetes. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia 2001 Oct;44(Suppl 3):B75-B80.
- 16. Zayed H. Epidemiology of diabetic ketoacidosis in Arab patients with type 1 diabetes: a systematic review. Int J Clin Pract 2016 Mar;70(3):186-195.
- 17. Habib HS. Frequency and clinical characteristics of ketoacidosis at onset of childhood type 1 diabetes mellitus in Northwest Saudi Arabia. Saudi Med J 2005 Dec;26(12):1936-1939.
- 18. Ahmed AM, Al-Maghamsi M, Al-Harbi AM, Eid IM, Baghdadi HH, Habeb AM. Reduced frequency and severity of ketoacidosis at diagnosis of childhood type 1 diabetes in Northwest Saudi Arabia. J Pediatr Endocrinol Metab 2016 Mar;29(3):259-264.
- 19. Abduljabbar MA, Aljubeh JM, Amalraj A, Cherian MP. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi Med J 2010 Apr;31(4):413-418.
- 20. Albishi L, Altoonisi M, Alblewi S, Osman R, Ahmed N, Fararjeh M. Clinical demographic patterns of type 1 diabetes in Saudi children in Tabuk City, 2000-2010. J Diabetes Mellitus 2017 May;7(2):41-54.
- 21. Al-Hayek AA, Robert AA, Braham RB, Turki AS, Al-Sabaan FS. Frequency and associated risk factors of recurrent diabetic ketoacidosis among Saudi adolescents with type 1 diabetes mellitus. Saudi Med J 2015 Feb;36(2):216-220.
- 22. Maldonado MR, Chong ER, Oehl MA, Balasubramanyam A. Economic impact of diabetic ketoacidosis in a multiethnic indigent population: analysis of costs based on the precipitating cause. Diabetes Care 2003 Apr;26(4):1265-1269.
- 23. Hamed ZS, Gawaly A, Abbas K, El Ahwal L. Epidemiology of infection as a precipitating factor for diabetic ketoacidosis at Tanta University Hospital. Tanta Med J 2017 Apr;45(2):68-72.
- 24. Azoulay E, Chevret S, Didier J, Barboteu M, Bornstain C, Darmon M, et al. Infection as a trigger of diabetic ketoacidosis in intensive care-unit patients. Clin Infect Dis 2001 Jan;32(1):30-35.
- Randall L, Begovic J, Hudson M, Smiley D, Peng L, Pitre N, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care 2011 Sep;34(9):1891-1896.
- Wright J, Ruck K, Rabbitts R, Charlton M, De P, Barrett T, et al. Diabetic ketoacidosis (DKA) in Birmingham, UK, 2000-2009: an evaluation of risk factors for recurrence and mortality. Br J Diabetes Vasc Dis 2009;9(6):278-282.
- 26. Rodin GM, Daneman D. Eating disorders and IDDM. A problematic association. Diabetes Care 1992 Oct;15(10):1402-1412.
- 28. Pinhas-Hamiel O, Hamiel U, Levy-Shraga Y. Eating disorders in adolescents with type 1 diabetes: challenges in diagnosis and treatment. World J Diabetes 2015 Apr;6(3):517-526.
- 29. Karges B, Neu A, Hofer SE, Rosenbauer J, Kiess W, Rütschle H, et al. Frequency and influencing factors of ketoacidosis at diabetes onset in children and adolescents–a long-term study between 1995 and 2009. Klin Padiatr 2011 Mar;223(2):70-73.
- 30. Chafe R, Albrechtsons D, Hagerty D, Newhook LA. Reducing episodes of diabetic ketoacidosis within a youth population: a focus group study with patients and families. BMC Res Notes 2015 Sep;8(1):395.
- 31. Curtis JR, To T, Muirhead S, Cummings E, Daneman D. Recent trends in hospitalization for diabetic ketoacidosis in ontario children. Diabetes Care 2002 Sep;25(9):1591-1596.
- 32. Brzezicki A, Mcgibbon AM. DKA management in the emergency room and length of stay. Can J Diabetes 2016 Oct;40(5):S44.
- 33. Babiker A, Aljahdali G, Alsaeed M, Almunif A, Mohamud M, Abid O, et al. Characteristics of admissions with diabetic ketoacidosis in a specialized children hospital: Missing insulin is still a challenge! 2020 [cited]. Available from: https://authorea.com/users/331920/articles/458409-characteristics-of-admissions-with-diabetic-ketoacidosis-in-a-specialized-children-hospital-missing-insulin-is-still-a-challenge?commit=36a67fee873e63287d3457daa4f1461a768e2178.