|
Abstract
Toxoplasmosis is caused by infection with the obligate intracellular parasite Toxoplasma gondii. Toxoplasmosis is generally a late complication of HIV infection and usually occurs in patients with CD4 + T-cell counts below 200/μl. Co-trimoxazole (trimethoprim plus sulfamethoxazole) is the most common drug used in India for the treatment of AIDS-associated cerebral toxoplasmosis. Other alternative drugs used for the treatment of cerebral toxoplasmosis are clindamycin plus pyrimethamine and clarithromycin with pyrimethamine.
A 30-year-old male known case of retroviral disease presented to Kasturba Medical College, India, with complaints of fever, headache and vomiting. Computed tomography scan of his brain showed irregular ring enhancing lesion in the right basal ganglia. Toxoplasma serology revealed raised IgG antibody levels. Based on the CT features and serology, diagnosis of cerebral toxoplasmosis was made. He was treated with clindamycin alone as he had historyof sulfonamide allergy. The patient was symptomatically better after 48 hours. After 21 days, repeat CT of brain was done which was normal. The patient showed good clinical improvement within 48 hours and the lesion resolved completely within 3 weeks. The authors recommend using clindamycin without pyrimethamine in resource poor settings and in patients who do not tolerate sulfa drugs.
Keywords: Cerebral toxoplasmosis; Clindamycin; HIV/AIDS.
Introduction
Toxoplasmosis is one of the most common causes of focal brain lesions in patients with acquired immune deficiency syndrome particularly in developing countries.1 The disease is treatable, most patients making a full recovery, but it is fatal if untreated. Pyrimethamine plus sulfadiazine, trimethoprim plus sulfamethoxazole, clindamycin plus pyrimethamine,2 and clarithromycin plus pyrimethamine are used to treat cerebral toxoplasmosis. Clindamycin plus pyrimethamine is principally used in patients who do not tolerate sulfonamides. There are limited published data on the use of clindamycin alone in the treatment of cerebral toxoplasmosis.
Case Report
A 30-year-old male presented to Kasturba Medical College, India, with complaints of fever, headache and vomiting of 7 days duration. He was diagnosed with retroviral disease one month back and was on antiretroviral drugs (stavudine, lamivudine, nevirapine). On examination, he was febrile and drowsy. There was no focal neurological deficit.
Laboratory investigations showed Hb 8.8 g/dL, total white blood cell count 2.2×109/L, ANC 0.8×109/L, platelet count 353×109/L, ESR 28 mm/1st hour. Peripheral smear showed dimorphic anemia with leukopenia. Serum electrolytes, blood sugar, renal and liver function tests were normal. Test for HIV-1 was reactive. His CD4+ count was 38 cells/μl. Chest X-ray and ultrasound of the abdomen were normal.
Computed tomography scan of the brain showed an irregular ring enhancing lesion in the right basal ganglia with surrounding marked white matter edema and mass effect, (Fig. 1). CSF analysis was not done (in view of significant edema and mass effect). Toxoplasma serology revealed raised IgG antibody levels of 326 IU/mL.
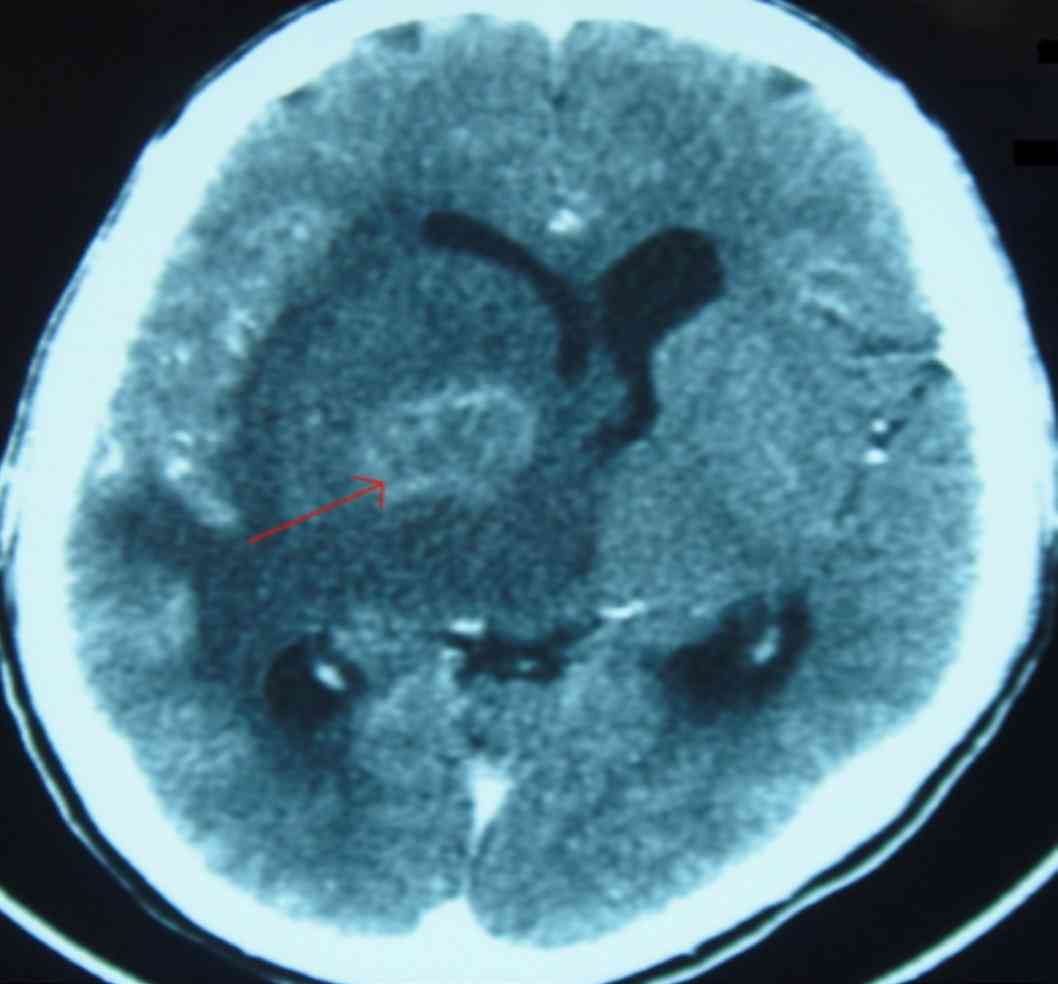
Figure 1: Brain CT scan showing irregular ring enhancing lesion in the right basal ganglia with surrounding marked white matter edema and mass effect.
The patient was treated with IV mannitol, clindamycin (600 mg thrice daily), and anticonvulsants. Antiretroviral drugs were continued. His symptoms improved gradually within 48 hours of admission. After 21 days, repeat CT of brain was done which was normal, (Fig. 2). The patient was discharged from hospital in an ambulatory state. He was advised to continue antiretroviral drugs and anticonvulsants. Trimethoprim-sulfamethoxazole was started (prophylactic dose) after following a sulfa desensitization protocol. He has been asymptomatic for the past 9 months.
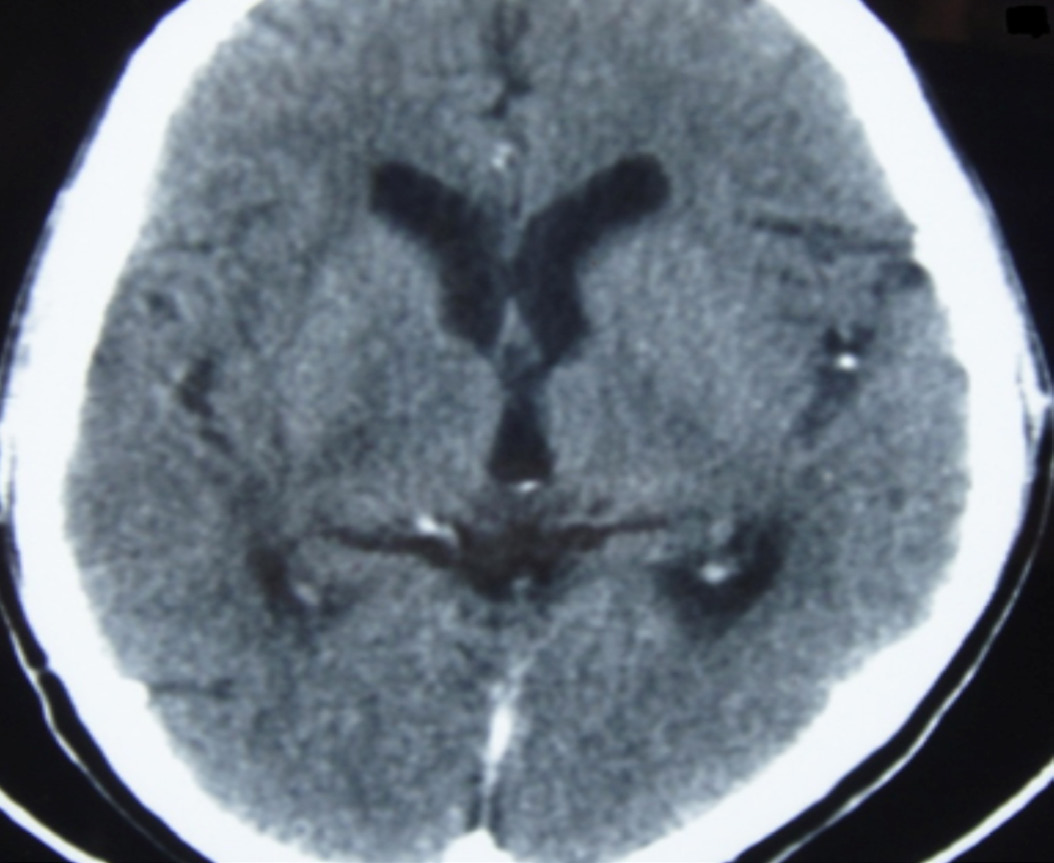
Figure 2: CT scan after 21 days of treatment.
Discussion
Human infection occurs via oral or transplacental route. The major clinical features of cerebral toxoplasmosis are headache, hemiparesis, speech disturbances, cerebellar dysfunction and cranial nerve palsies.
CT scan typically reveals bilateral, multiple, hypodense ring-enhancing lesions with surrounding edema in 60% to 70% of patients. Lesions can be solitary in 27% of patients.3 The patient had a solitary lesion. If the CT scan is normal during initial screening, MRI is recommended because it is more sensitive and will detect additional lesions in some cases.4 The patient had financial problems so MRI brain was not done. In addition to toxoplasmosis, the differential diagnoses of single or multiple enhancing mass lesions in the HIV-infected patient include primary CNS lymphoma, tuberculosis, and fungal or bacterial abscesses. About 97% of patients with cerebral toxoplasmosis have toxoplasma IgG antibodies and the levels are usually over 1:256.5 IgG antibody levels in this patient were 326 IU/mL.
Cerebral toxoplasmosis is generally treated empirically in AIDS patients with compatible neuro-imaging studies and positive serology. Brain biopsy should only be considered in patients with negative toxoplasma serology and who do not respond to treatment.6
Trimethoprim/sulfamethoxazole is an alternative treatment for toxoplasma encephalitis because it is inexpensive and as effective as pyrimethamine-sulfadiazine,7 which is the first line drug. At our centre, we use trimethoprim/sulfamethoxazole to treat AIDS associated cerebral toxoplasmosis. Pyrimethamine alone is available only in a few institutions in India and it was not used in this patient. The patient had a history of sulfa allergy so he was treated with intravenous clindamycin (600 mg thrice daily) for 3 weeks. Yapar et al.8 have used clindamycin alone to treat cerebral toxoplasmosis but it took 10 months for complete radiological clearance of the lesions. While Roemer et al.9 used clindamycin to treat a patient with cerebral toxoplasmosis but the patient died. The potential use of clindamycin as a single agent has not been established in randomized clinical trials. The patient in this case showed good clinical improvement within 48 hours and the lesion resolved completely within 3 weeks. A positive response to treatment can be demonstrated both clinically and radiologicaly. Patil et al.10 repeated CT scan after 21 days of treatment to demonstrate response to antitoxoplasma therapy. Beraud et al.11 used clinical and radiological criteria to demonstrate response to treatment.
Conclusion
Cerebral toxoplasmosis can be treated with clindamycin without pyrimethamine in resource poor settings countries and in patients who do not tolerate sulfa drugs.
Acknowledgements
The authors reported no conflict of interest and no funding was received in this work.
References
1. Shankar SK, Mahadevan A, Satishchandra P, Kumar RU, Yasha TC, Santosh V, et al. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J Med Res 2005 Apr;121(4):468-488.
2. Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, et al; The California Collaborative Treatment Group. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann Intern Med 1992 Jan;116(1):33-43.
3. Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 1992 Dec;327(23):1643-1648.
4. Ciricillo SF, Rosenblum ML. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg 1990 Nov;73(5):720-724.
5. Skiest DJ, Erdman W, Chang WE, Oz OK, Ware A, Fleckenstein J. SPECT thallium-201 combined with Toxoplasma serology for the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect 2000 May;40(3):274-281.
6. Mathews C, Barba D, Fullerton SC. Early biopsy versus empiric treatment with delayed biopsy of non-responders in suspected HIV-associated cerebral toxoplasmosis: a decision analysis. AIDS 1995 Nov;9(11):1243-1250.
7. Torre D, Speranza F, Martegani R, Zeroli C, Banfi M, Airoldi M. A retrospective study of treatment of cerebral toxoplasmosis in AIDS patients with trimethoprimsulphamethoxazole. J Infect 1998;17:15-18 .
8. Yapar N, Erdenizmenli M, Oğuz VA, Cakir N, Yüce A. Cerebral toxoplasmosis treated with clindamycin alone in an HIV-positive patient allergic to sulfonamides. Int J Infect Dis 2005 Jan;9(1):64-66.
9. Roemer E, Blau IW, Basara N, Kiehl MG, Bischoff M, Günzelmann S, et al. Toxoplasmosis, a severe complication in allogeneic hematopoietic stem cell transplantation: successful treatment strategies during a 5-year single-center experience. Clin Infect Dis 2001 Jan;32(1):E1-E8.
10. Patil HV, Patil VC, Rajmane V, Raje V. Successful treatment of cerebral toxoplasmosis with cotrimoxazole. Indian J Sex Transm Dis 2011 Jan;32(1):44-46.
11. Béraud G, Pierre-François S, Foltzer A, Abel S, Liautaud B, Smadja D, et al. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994-2006. Am J Trop Med Hyg 2009 Apr;80(4):583-587.
|