Acute leukemias (AL) are a heterogeneous group of hematological malignancies with the presence of 20% or more blasts in peripheral blood and bone marrow. They present with varying clinical, morphologic, immunologic, and molecular features. Malignant cells display characteristic patterns of surface antigenic expression. Immunophenotyping of leukemia cells results in broad classification of in to acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), and mixed phenotypic acute leukemia (MPAL) based on the expression of different subsets of surface molecules now defined as cluster of differentiation (CD) antigens. Flow cytometry is used to study the antigenic expression of CD markers on leukocytes.1
Aberrant phenotypes in AL are defined as patterns of antigen expression on neoplastic cells different from the process of normal hematopoietic maturation.
Aberrant expressions of antigen in AL include lineage infidelity (lymphoid marker expression in myeloid blast cells like CD7, CD19, CD79a, CD10, CD2, CD5, CD3 expression) as well as asynchronous antigen expression (coexistence of early and late markers in one cell like CD34 and CD15 in AML). Aberrant expression also includes antigen over expression, which is abnormally increased expression of certain antigen per cell, aberrant light scatter properties, and absence of lineage specific antigens, which involves absence of expected antigen expression (CD13 and CD33 on myeloid blasts). In ALL these aberrancies include cross-lineage antigen expression (expression of myeloid antigens in ALL, B-lineage antigens in T-ALL or T-lineage antigens in B-ALL).2
Aberrant phenotypes can be used for minimal residual disease (MRD) detection and monitoring. MRD is defined as persistence of leukemic cells after chemotherapy that cannot be identified with routine morphologic evaluation.3
With various modifications in flow cytometric instrument and availability of wide range of antibodies and fluorochromes have changed our perception in identification of normal cell population and aberrancies present on leukemic cells. Our study sought to evaluate the occurrence of aberrant phenotype in newly diagnosed cases of AL.
Methods
This prospective study conducted in the Department of Pathology of a tertiary care centre in North India and included 100 newly diagnosed cases of AL during the period of June 2017 to December 2018. Diagnosis of AL was done based on morphological examination of Leishman stained peripheral blood smears and bone marrow aspiration smears, cytochemistry, and flow cytometric immunophenotyping.
Flow cytometric analysis was done using the monoclonal antibody panel of AL on peripheral blood/bone marrow using a standard stain-lyse-wash method. Listmode data was acquired on FACSCanto II Flow cytometer (Becton Dickinson, San Jose, CA) and analyzed by FACSDiva software. Results were obtained by gating the blasts cells with side scatter (SSC) versus forward scatter (FSC) followed by SSC versus CD45 gating. For surface antigen, marker positivity was considered when > 20% blasts cells were positive and > 10% blast cell positivity was considered for cytoplasmic antigen.
All the quality control measures were undertaken before starting the procedure such as checking the system pressure and vacuum gauges, checking the optical alignment of red and blue laser, titration of antibodies for fluorescence standardization, color compensation using seven color set-up beads of instrument and verification of antibody integrity using quality control protocol.4
Required approval from institutional ethical committee of University of Health Sciences was taken. Informed consents were obtained from all participants after explanation of the study. After hematological study and flow cytometry, the biomedical waste generated during the procedure were discarded as per biomedical waste (management and handling) rules.5
SPSS statistical software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) was used for statistical analysis. Quantitative (continuous) variables were expressed as mean. Categorical variables were expressed as frequencies and percentage and nominal categorical data between the groups were compared using chi-squared test. In all statistical tests, a p-value < 0.500 was considered significant. Association of aberrant antigen expression in cases was correlated with clinical, morphological, and cytochemistry findings using the chi-square test.
Table 1: Clinical features of acute leukemia cases, n (%).
|
Pallor |
49 (92.5) |
37 (86.0) |
4 (100) |
90 (90.0) |
|
Fever |
35 (66.0) |
38 (88.4) |
3 (75.0) |
76 (76.0) |
|
Organomegaly (hepatosplenomegaly) |
38 (71.7) |
24 (55.8) |
3 (75.0) |
65 (65.0) |
|
Bleeding |
11(20.8) |
12 (27.9) |
2 (50.0) |
25 (25.0) |
|
Lymphadenopathy |
2 (3.8) |
16 (37.2) |
3 (75.0) |
21 (21.0) |
|
Rash |
4 (7.5) |
5 (11.6) |
2 (50.0) |
11 (11.0) |
AML: acute myeloid leukemia; ALL: acute lymphoid leukemia; MPAL: mixed phenotypic acute leukemia.
Results
Out of total of 100 patients with AL, 53 cases were categorized as AML, 43 cases as ALL, and four cases as MPAL. ALL was subtyped into B-ALL and T-ALL with 38 cases (88.4%) and five cases (11.6%) in each, respectively. Fever (76.0%) was the most commonly presenting complaint followed by bleeding (25.0%), and gum hypertrophy (10.0%). On examination, pallor was observed in 90.0% cases, organomegaly (hepatosplenomegaly) in 65.0%, and lymphadenopathy in 21.0% of cases. Clinical features in AL are shown in Table 1.
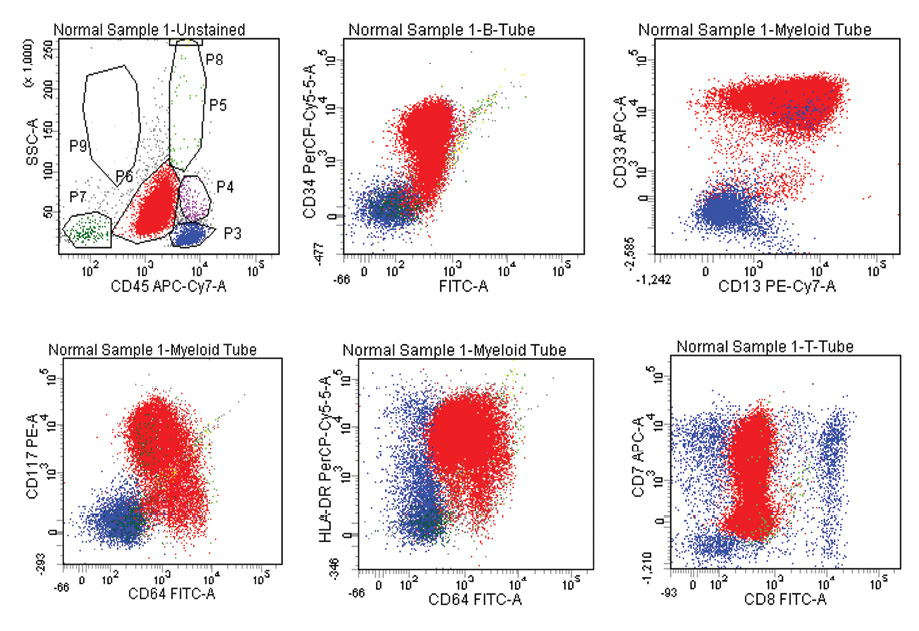
Figure 1: Acute myeloid leukemia with aberrant expression of CD7, Blasts are positive for CD13, CD33, CD34, CD117, CD64, HLA-DR, and CD7. Dim positive for CD45 and negative for CD8.
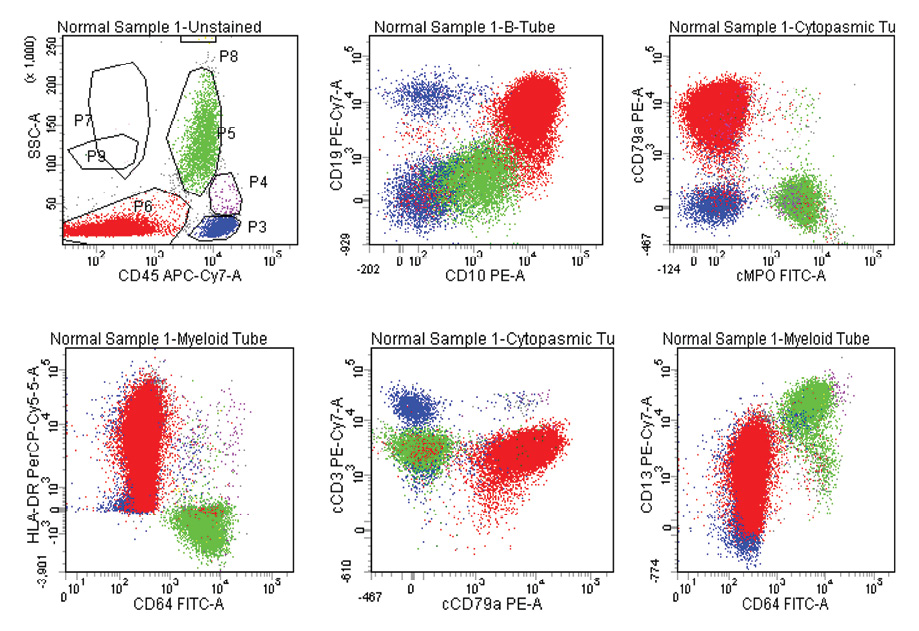
Figure 2: B-ALL with aberrant expression of CD13. Blasts are positive for CD19, CD10, cCD79a, HLA-DR, and CD13, negative to Dim, positive for CD45, and negative for cMPO, CD64, CD33, and cCD3.
Table 2: Aberrant antigen expression in acute myeloid leukemia (AML) cases (n = 14).
|
T + Ly AML |
|
|
|
CD7 |
9/14 |
17.0 |
|
B+ Ly AML |
|
|
|
CD19 |
3/14 |
5.7 |
Table 3: Aberrant antigen expression in B-ALL cases (n = 38).
|
CD34, CD20 |
20 |
52.6 |
|
CD13 |
19 |
50.0 |
|
CD117 |
2 |
5.3 |
|
CD33 |
1 |
2.6 |
|
CD13, CD33 |
1 |
2.6 |
|
CD13, CD5 |
1 |
2.6 |
|
CD117, C7 |
1 |
2.6 |
|
CD7 |
1 |
2.6 |
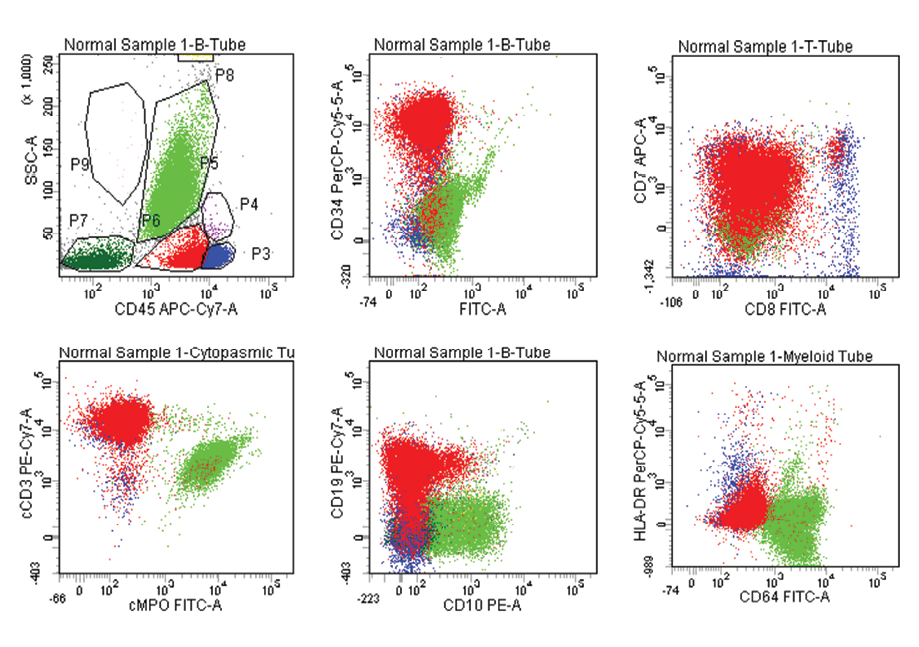
Figure 3: T- ALL with aberrant expression of CD19. Blasts are positive for CD7, CD34, CD8, CD64, cCD3, CD19, Dim positive for CD45, negative for CD64, CD10, cMPO.
Table 4: Aberrant antigen expression in T-ALL cases (n = 5).
|
CD117 |
1 |
20.0 |
|
CD19 |
1 |
20.0 |
|
CD20 |
1 |
20.0 |
Cytochemical staining with myeloperoxidase (MPO) and Sudan black B (SBB) coupled together was positive in 41 (77.4%) cases of AML. Periodic acid-Schiff (PAS) staining was positive in 36 (83.7%) cases of ALL. MPO and SBB were positive in one (25.0%) case, and PAS was positive in one (25.0%) case of MPAL. Two (50.0%) cases of MPAL showed positive expression of MPO, SBB, and PAS.
CD45 is an immunological marker used for gating purpose for the identification of blast cells by flow cytometry. In AML (53.0%), CD33 and CD13 were the most commonly expressed (98.1%) antigens followed by MPO (88.7%), CD117 (73.6%), CD64 (64.2%), and CD34 (64.2%). In B-ALL (38.0%), CD19 was expressed in all (100%) cases followed by CD10 (94.7%), cCD79a (92.1%), Tdt (86.8%), CD20 (84.2%), and CD34 (65.8%). In T-ALL (5.0%), cCD3, CD7, and CD5 were expressed in all (100%) cases followed by CD4 (80.0%) and CD8 (20.0%). In MPAL (4.0%), all were B/myeloid with 100% expression of CD13, CD33, MPO, CD19, CD79a, CD34, and HLA-DR.
In AML, 14 (26.4%) out of 53 cases showed aberrant lymphoid antigen expression. CD7 was the most common aberrant antigen expressed in nine (17.0%) cases followed by CD19 in three (5.7%) cases and CD10 in two (3.8%) cases. Co-expression of HLA-DR and CD34 was seen in about two-thirds of patients (64.0%) with AML in our study [Figure 1]. In B-ALL, 21 (55.3%) out of 38 cases expressed aberrant antigens, with CD13 (50.0%) being the most common expressed aberrant antigen followed by CD33 (2.6%), CD7 (2.6%), and CD5 (2.6%). Asynchronous dual expression of CD34 and CD20 was found in 20 (52.6%) cases of B-ALL [Figure 2]. In T-ALL, four (80.0%) out of five cases expressed aberrant antigens with CD117, CD19, CD20, and CD10 expressed in one case each of T-ALL. Aberrant expression of CD markers in AML, B-ALL and T-ALL are shown in Table 2, 3, 4, and Figure 3.
Discussion
In India, ALL and AML are 35% and 15% of all hematological malignancies, respectively.6 We performed this study to categorize by cytomorphology (lymphoid and myeloid), correlate the immunophenotyping profile with the cytomorphological category, and evaluate the frequency of aberrant phenotype by flow cytometry.
There is variable expression of lymphoid antigen in AML based on a range of markers studied, the total number of samples, and aberrancy criteria. In our study, 53 patients were diagnosed as AML, out of which 14 (26.4%) showed aberrant antigen expression. CD7 was the most common aberrant antigen expressed in nine (17.0%) cases, followed by CD19 in three (5.7%) cases, and CD10 in two (3.8%) cases.
Our findings are in agreement to the previous studies4,7–9 who also reported CD7 as the most common aberrant lymphoid antigen. CD7 is an activation and adhesion molecule and is expressed by T cells, NK cells, and stem cells. CD7 expression likely to represent an origin from early stem cells in myeloid development and is associated with immaturity markers CD34 and HLA-DR, antigen receptor gene rearrangements and lower chances of complete remission, and overall poor prognosis.7
There is no consensus on the clinical relevance of lymphoid antigen expression in AML. Some studies7,9 reported Ly+AML to be associated with poor prognosis, but some reported it to be associated with favorable prognosis, whereas others suggest it to be of no prognostic value.10
In our study, aberrant phenotypes showed poor prognostic factors in AML cases, although it did not achieve statistical significance, similar to the previous studies.7–9,11 The low frequency of aberrant antigen expression in AML in our study compared to published literature may be related to technical issues (differences in the number of patients tested for those markers), difference in laboratory protocols, or due to ethnical variation.
The degree of myeloid antigen expression in ALL cases varied from 4.3%–64%.2.12.13 This variation may be due to clones of monoclonal antibodies used having different binding characteristics, variation in the threshold for antigen positivity, and different flow cytometry methods used in sample processing, make of instruments, and reagents.
In our study, 21 (55.3%) cases of total B-ALL expressed aberrant antigens, with CD13 (50.0%) being the most commonly expressed followed by CD33 (2.6%), CD7 (2.6%), and CD5 (2.6%). Our findings were inconsistent with Sharma et al (42.5%).12 Salem et al,13 reported a slightly lower incidence of 10.5%, while Seegmiller et al,2 reported a high incidence of 86.5% aberrant antigen expression in B-ALL.
Most of the studies,2,4,12–14 reported CD13 and CD33 as the most common aberrant myeloid antigen in ALL, which was also observed in our study. However, the clinical significance of expression of myeloid antigen in B-ALL is variable in the literature. Belurkar et al,4 and Seegmiller et al,2 did not find any significant effect of aberrant myeloid antigen expression on clinical presentation, relapse rate, and survival except their role in MRD detection in B-ALL.
Sharma et al,12 found that the adult My+ALL group was associated with statistically significant lower white blood cell count and lower peripheral blast count. In contrast, in pediatric cases, it was associated with lymphadenopathy, lower blast count, and higher expression of CD34. Rodríguez-Rodríguez et al,9 found a statistically significant association between myeloid markers in adult ALL with inferior disease-free survival and shorter survival. In contrast to these studies, Lopes et al,14 found that the platelet count was significantly lower in the group without aberrant myeloid expression and may indicate a greater risk of bleeding during treatment in this group.
In our study, B-ALL patients with aberrant antigen expression showed an association with poor prognostic factors, although this did not achieve statistical significance. In T-ALL, four (80.0%) of our cases expressed aberrant antigens, with CD117, CD19, CD20, and CD10 being expressed in one case each of T-ALL, which was higher than the published literature.1,12,13 The higher incidence of aberrancy in our study could be due to the small number of cases in this group.
We could not correlate treatment response parameters to comment upon the overall prognostic significance of expression of aberrant markers in AL, which is a limitation of our study. However, evaluation of immunophenotypic aberrancies’ expression remains valuable for more precise characterization of the leukemic population and can help make alternate therapeutic decisions and monitoring of MRD during the disease.
Conclusion
Simultaneous application of multiple techniques are used nowadays for the diagnosis and classification of leukemia. Correlation of cytomorphology, cytochemistry, FCM, cytogenetics, and molecular techniques is required to assign the diagnostic sample to the correct entity.
Flow cytometry is of great help in the diagnosis of AL, particularly in ALL for lineage assignment and in classifying the unclassifiable cases based on morphology and cytochemistry. MPA also helps in detecting aberrant antigen expression.
Our results point to the need for a more extensive and elaborated study to evaluate the potential prognostic significance of aberrant marker expression in AL. The presence of aberrancies strongly supports the continued use of FCM in the diagnosis and monitoring of AL.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Jha SC, Muzaffar MA, Singh A, Kumar A, Raza S, Dwivedi RP. Flowcytometric evaluation and morphological and cytochemical correlation of 150 cases of Acute Leukaemia. Int J Biol Adv Res 2015;6(12):844-852.
- 2. Seegmiller AC, Kroft SH, Karandikar NJ, McKenna RW. Characterization of immunophenotypic aberrancies in 200 cases of B acute lymphoblastic leukemia. Am J Clin Pathol 2009 Dec;132(6):940-949.
- 3. Al-Mawali A, Gillis D, Hissaria P, Lewis I. Incidence, sensitivity, and specificity of leukemia-associated phenotypes in acute myeloid leukemia using specific five-color multiparameter flow cytometry. Am J Clin Pathol 2008 Jun;129(6):934-945.
- 4. Belurkar S, Mantravadi H, Manohar C, Kurien A. Correlation of morphologic and cytochemical diagnosis with flowcytometric analysis in acute leukemia. J Cancer Res Ther 2013 Jan-Mar;9(1):71-79.
- 5. National Accreditation Board for Hospitals and healthcare providers. Biomedical waste management rules 2016[Internet]. New Delhi: Gazette of India, Extraordinary, Part II, Section 3, Subsection (i); 2016 Mar 28 [updated 2016 Aug 03; cited 2016 Aug 20].Available from: http;://nabh.co/announcement /bmw_rules_2016.pdf.
- 6. Singh G, Parmar P, Kataria SP, Singh S, Sen R. Spectrum of acute and chronic leukaemia in tertiary care hospital, Haryana,India. Int J Med Res Sci. 2016;4(4):1115-1118.
- 7. Saxena A, Sheridan DP, Card RT, McPeek AM, Mewdell CC, Skinnider LF. Biologic and clinical significance of CD7 expression in acute myeloid leukemia. Am J Hematol 1998 Aug;58(4):278-284.
- 8. Bahia DM, Yamamoto M, Chauffaille MdeL, Kimura EY, Bordin JO, Filgueiras MA, et al. Aberrant phenotypes in acute myeloid leukemia: a high frequency and its clinical significance. Haematologica 2001 Aug;86(8):801-806.
- 9. Rodríguez-Rodríguez S, Pomerantz A, Demichelis-Gómez R, Barrera-Lumbreras G, Barrales-Benítez OV, Lopez-Karpovitch X, et al. Impact of Aberrant Antigens in the Outcome of Patients with Acute Leukemia at a Referral Institution in Mexico City. Rev Invest Clin 2016 Nov-Dec;68(6):305-313.
- 10. Aparna SK, Sharmilla M. Aberrant phenotypes in acute myeloid leukaemia in India. Int J Adv Med. 2018;5(2):361-365.
- 11. Bhushan B, Chauhan PS, Saluja S, Verma S, Mishra AK, Siddiqui S, et al. Aberrant phenotypes in childhood and adult acute leukemia and its association with adverse prognostic factors and clinical outcome. Clin Exp Med 2010 Mar;10(1):33-40.
- 12. Sharma M, Sachdeva MS, Varma N, Varma S, Marwaha RK. Characterization of immunophenotypic aberrancies in adult and childhood acute lymphoblastic leukaemia, Jr of Can Res andTher. 2016;12(2):620-626.
- 13. Salem DA, Abd El-Aziz SM. Flowcytometric immunophenotypic profile of acute leukemia: mansoura experience. Indian J Hematol Blood Transfus 2012 Jun;28(2):89-96.
- 14. Lopes TC, Andrade KN, Camelo NL, Rodrigues VP, Oliveira RA. Influence of aberrant myeloid expression on acute lymphoblastic leukemia in children and adolescents from Maranhão, Brazil. Genet Mol Res 2014 Dec;13(4):10301-10307.