Streptococcus pyogenes is a spherical Gram-positive, β-hemolytic, catalase-negative bacterium arranged in chains, and a facultative anaerobe.1,2 S. pyogenes cell wall contains two group- and type-specific antigens (i.e., Lancefield group A antigen (Group A) and M protein).1,2 Lancefield group A Streptococcus (GAS) is a highly prevalent human pathogen whose asymptomatic colonization can occur in the oropharynx of healthy school-aged children and young adults.2 Common and important diseases associated with S. pyogenes are classified into two groups, including suppurative and non-suppurative infections. Suppurative infections are manifested as pharyngitis, scarlet fever, pyoderma, erysipelas, cellulitis, necrotizing fasciitis (streptococcal gangrene), streptococcal toxic shock syndrome, puerperal sepsis, lymphangitis, and pneumonia. Non-suppurative post-infectious immune-mediated infections include acute rheumatic fever, rheumatic heart disease, and acute post-streptococcal glomerulonephritis.2,3 Person-to-person transmission of GAS infection occurs via respiratory droplets.2 In 2005, the World Health Organization estimated that GAS infections’ global prevalence is approximately 18.1 million, with 1.78 million new cases annually.4 The report adds that GAS infections are the ninth infection causing death in humans, especially in developing countries, with over 517 000 deaths worldwide annually.3,4 Therefore, appropriate therapy using antibiotics is an important strategy to be implemented. Recommended antibiotic regimens for the treatment of GAS infections are oral administration of penicillin V and amoxicillin and intramuscular benzathine penicillin G for individuals without penicillin allergy, and also narrow-spectrum cephalosporins including oral cephalexin and cefadroxil and oral clindamycin, azithromycin, and clarithromycin for individuals with penicillin allergy.2,5,6 However, antibiotic resistance of GAS is well-documented and worrisome, and thus epidemiological studies and global surveillance remain a high priority. Although few studies have investigated drug resistance of GAS in different cities of Iran, there has been no comprehensive study on the overall prevalence of GAS resistance in Iran. Therefore, the current systematic review and meta-analysis’s main objective was to assess antibiotic resistance of GAS strains isolated from Iranian children.
Methods
We conducted a systematic review and meta-analysis on GAS antibiotic resistance in Iranian children following the PRISMA international guideline.7 Both national (Scientific Information Database) and international (ISI Web of Knowledge, PubMed, Scopus, and Google Scholar) electronic databases were searched up to 20 March 2019. Additionally, to ensure a comprehensive search and avoid missing any related articles, the reference lists of all included articles were checked manually. Streptococcus pyogenes, S. pyogenes, group A Streptococcus, or GAS and children along with Iran were search terms in the English language. The same terms were applied to find Persian studies. Literature search, along with article selection and data collection, were performed by two reviewers independently.
We screened titles, abstracts, and full-texts of publications to select eligible articles according to the inclusion or exclusion criteria. We included studies conducted in Iran, studies reporting the prevalence of GAS antibiotic resistance isolated from children, and articles published in national and international languages. We also excluded studies simultaneously published in Persian and English languages with similar results, review articles, abstracts and letters, cross-sectional studies with incomplete data, studies reporting the prevalence of GAS colonization, and studies reporting GAS antibiotic resistance in adults.
We abstracted data from eligible studies with information on the first author name, location and year of the study, number of GAS strains, antimicrobial susceptibility testing, and the number of GAS strains resistant to different antibiotics [Table 1]. Additionally, the quality assessment of included studies was done using the Joanna Briggs Institute (JBI) critical appraisal checklist for studies reporting prevalence data. Cross-sectional studies were considered high-quality when they received scores > 5 based on the JBI criteria. The JBI checklist assessed the presence of basic data including the target population, the sample size, statistical analysis, and identification methods.
Table 1: Profiles of included studies in the meta-analysis.
|
Khosravi8 |
Ahvaz |
2012-2013 |
8 |
Children |
25 |
Disk diffusion |
0 |
ND |
1 |
2 |
ND |
1 |
ND |
3 |
ND |
0 |
11 |
4 |
ND |
ND |
ND |
ND |
|
Ghaffari9 |
East Azerbaijan |
2010 |
8 |
Children |
67 |
Disk diffusion |
0 |
59 |
5 |
ND |
ND |
28 |
ND |
ND |
35 |
54 |
15 |
ND |
33 |
ND |
62 |
47 |
|
Nabipour10 |
Kerman |
- |
8 |
Children |
57 |
Disk diffusion |
57 |
50 |
7 |
ND |
ND |
ND |
ND |
ND |
ND |
46 |
2 |
ND |
28 |
ND |
52 |
40 |
|
Kamaly11 |
Qazvin |
- |
7 |
Children |
44 |
Disk diffusion |
0 |
0 |
0 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
4 |
ND |
44 |
ND |
|
Mohseni-
moghaddam12 |
Rafsanjan |
2009 |
8 |
Children |
7 |
Disk diffusion |
2 |
1 |
0 |
0 |
ND |
ND |
ND |
ND |
ND |
0 |
0 |
ND |
0 |
ND |
ND |
ND |
|
Ardalan13 |
Sanandaj |
2014 |
4 |
Children |
40 |
Disk diffusion |
0 |
ND |
8 |
4 |
1 |
3 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Kalantar14 |
Sanandaj |
2010 |
8 |
Children |
68 |
Disk diffusion |
10 |
ND |
10 |
ND |
ND |
8 |
ND |
1 |
ND |
16 |
9 |
ND |
16 |
11 |
5 |
2 |
|
Sayyahfar15 |
Tehran |
2010-2013 |
8 |
Children |
59 |
Disk diffusion
Broth dilution |
0 |
ND |
22 |
30 |
22 |
9 |
9 |
7 |
10 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Parvizi16 |
Tehran |
2011 |
8 |
Children |
24 |
Disk diffusion |
0 |
ND |
0 |
0 |
ND |
0 |
ND |
ND |
ND |
ND |
0 |
0 |
3 |
0 |
ND |
ND |
|
Jasir17 |
Tehran and
Guilan |
1995-1997 |
5 |
Children |
1335 |
Agar dilution |
0 |
ND |
3 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
560 |
ND |
ND |
ND |
|
Nourouzi18 |
Zahedan |
ND |
8 |
Children |
76 |
Disk diffusion |
6 |
15 |
2 |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
PEN: penicillin; AMX: amoxicillin; ERY: erythromycin; AZM: azithromycin; CLR: clarithromycin; CLI: clindamycin; RIF: rifampicin; CRO: ceftriaxone; CFM: cefixime; AMP: ampicillin; VAN: vancomycin; CHL: chloramphenicol; TET: tetracycline; CTX: cefotaxime; TMP/SXT: trimethoprim/sulfamethoxazole; GEN: gentamicin; JBI: the Joanna Briggs Institute critical appraisal checklist; ND: not determined; AST: antimicrobial susceptibility testing.
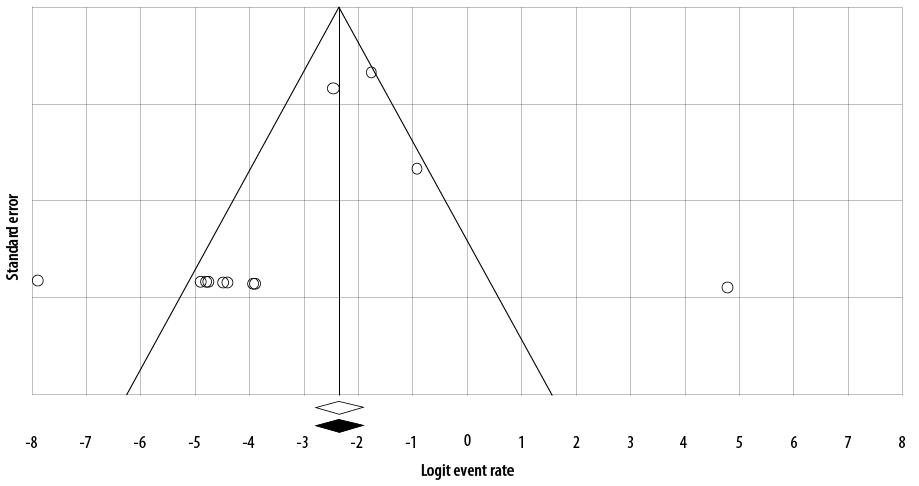
Figure 1: Funnel plot of the meta-analysis on the prevalence of antibiotic resistance of GAS to penicillin in Iran.
We used the I2 statistic and the chi-squared test with the Cochrane Q statistic to assess heterogeneity among studies. At high heterogeneity, I2 > 25% and p < 0.100, the prevalence of GAS antibiotic resistance was calculated using random-effects models. Possible publication bias evaluated with a funnel plot
[Figure 1]. If the funnel plot had an asymmetric shape, it was considered that there was some evidence of publication bias among included studies .
All statistical analyses were performed using Comprehensive Meta-Analysis software version 2.2 (Biostat, Englewood, NJ). The percentage of GAS strains’ resistance and its 95% confidence intervals (CIs) were estimated for each antibiotic.
Results
The literature search process is shown in Figure 2. Among the collected reports, a total of 12 studies met our inclusion criteria and were included in the meta-analysis for the analysis of GAS antibiotic resistance in Iranian children. Children with different GAS infections under the age of 15 years (2–15 years) were under study. Out of these 12 studies, five were in English, and the rest were in Persian, which reported GAS antibiotic resistance from Ahvaz, East Azerbaijan, Guilan, Kerman, Qazvin, Rafsanjan, Sanandaj, Tehran, and Zahedan [Table 2]. Study quality was also assessed based on the JBI criteria. As shown in Table 1, the included articles’ minimum score in the JBI criteria was 4.
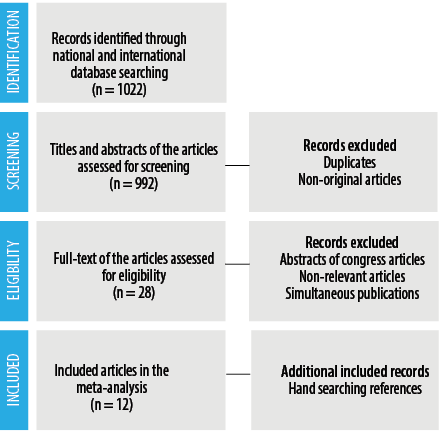
Figure 2: Literature search process.
Table 2: Antimicrobial resistance profiles of group A Streptococcus strains in Iranian children.
|
Penicillin |
4.2 |
95% CI: 1.2–13.3; I2 = 82.2%; Q = 61.9; p < 0.001 |
|
Amoxicillin |
38.3 |
95% CI: 7.6–82.3; I2 = 95.4%; Q = 87.1; p < 0.001 |
|
Erythromycin |
5.4 |
95% CI: 2.1–13; I2 = 88.9%; Q = 99.4; p < 0.001 |
|
Azithromycin |
12.0 |
95% CI: 2.7–39.8; I2 = 85.9%; Q = 28.4; p < 0.001 |
|
Clarithromycin |
12.6 |
95% CI: 0.7–75.5; I2 = 88.8%; Q = 9.0; p < 0.001 |
|
Clindamycin |
12.4 |
95% CI: 5.1–27.3; I2 = 83.8%; Q = 30.9; p < 0.001 |
|
Rifampicin |
15.3 |
- |
|
Ceftriaxone |
8.1 |
95% CI: 3.0–20.1; I2 = 53.5%; Q = 4.3; p = 0.110 |
|
Cefixime |
17.6 |
95% CI: 3.6–55; I2 = 91.8%; Q = 24.4; p < 0.001 |
|
Ampicillin |
36.9 |
95% CI: 10.4–74.6; I2 = 93.7%; Q = 64.3; p < 0.001 |
|
Vancomycin |
14.1 |
95% CI: 6.2–28.9; I2 = 76.4%; Q = 21.2; p < 0.001 |
|
Chloramphenicol |
8.4 |
95% CI: 1.2–41.7; I2 = 53.1%; Q = 2.1; p = 0.140 |
|
Tetracycline |
30.4 |
95% CI: 20.7–42.3; I2 = 82.7%; Q = 34.8; p < 0.001 |
|
Cefotaxime |
8.8 |
95% CI: 1.2–42.4; I2 = 57.4%; Q = 2.3; p = 0.120 |
|
Trimethoprim/sulfamethoxazole |
82.8 |
95% CI: 21.2–98.9; I2 = 96.3%; Q = 82.5 p < 0.001 |
|
Gentamicin |
39.6 |
95% CI: 10.7–78.3; I2 = 94.1%; Q = 34.2; p < 0.001 |
|
Ofloxacin |
11.9 |
95% CI: 0.6–73.4; I2 = 78.6%; Q = 4.6; p = 0.030 |
|
Carbenicillin |
28.3 |
- |
|
Ciprofloxacin |
3.1 |
- |
|
Imipenem |
6.1 |
- |
|
Cephalothin |
18.2 |
95% CI: 9.2–32.9; I2 = 64.2%; Q = 8.3; p = 0.030 |
|
Tobramycin |
57.6 |
95% CI: 43.4–70.7; I2 = 60%; Q = 2.5; p = 0.110 |
|
Kanamycin |
49.3 |
95% CI: 10.8–88.7; I2 = 95.3%; Q = 21.3; p < 0.001 |
|
Cloxacillin |
79.0 |
95% CI: 71.0–85.3; I2 = 0.0%; Q = 0.0; p = 0.980 |
|
Cephalexin |
12.9 |
95% CI: 0.9–69.7; I2 = 89.8%; Q = 19.6; p < 0.001 |
|
Cefazolin |
10.7 |
95% CI: 0.2–87.6; I2 = 93.8%; Q = 16.1; p < 0.001 |
CI: confidence interval.
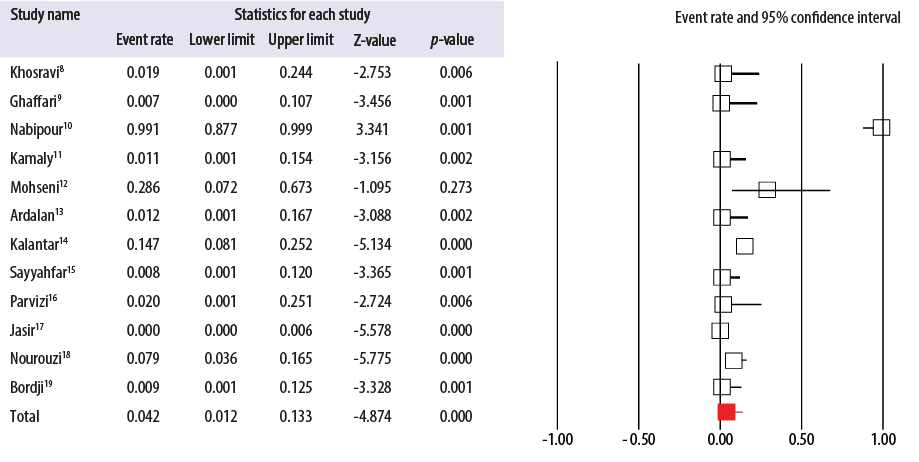
Figure 3: Forest plot of the meta-analysis on the prevalence of antibiotic resistance of group A Streptococcus to penicillin in Iran.
As shown in Table 1, Disk diffusion, broth dilution, and agar dilution were the tests that included studies used to determine GAS antimicrobial susceptibility in Iran [Table 1]. The GAS strains in Iran were mostly susceptible to ciprofloxacin 3.1%, penicillin 4.2%, erythromycin 5.4%, followed by imipenem 6.1%, ceftriaxone 8.1%, chloramphenicol 8.4%, and cefotaxime 8.8%. The highest resistance rate was reported against amoxicillin-clavulanic acid 89.5%, trimethoprim/sulfamethoxazole 82.8%, followed by cloxacillin 79.0% and tobramycin 57.6% [Table 2].
Discussion
The most common bacterial strain responsible for acute pharyngitis in children (20%–30%) and adults (5%–15%) is GAS.5 Therefore, accurate diagnosis along with proper antimicrobial treatment of pharyngitis is important to prevent non-suppurative post-infectious disorders of acute rheumatic fever and post-streptococcal glomerulonephritis as well as transmission of the organism.5 Patients with acute GAS pharyngitis can be treated with penicillin or amoxicillin, which have equal efficacy, for 10 days as the first-line treatment in non-allergic individuals due to the rare incidence of GAS resistant strains.5 For this reason, performing penicillins and other β-lactams susceptibility testing is not recommended by the US Food and Drug Administration.20 Other advantages are safety, modest cost, low side effects, and narrow-spectrum activity.5 However, the rate of streptococcal infection treatment failure with penicillin has been increasing in recent years and had reached 40%.21 GAS intracellular persistence and penicillin tolerance due to penicillin’s weak ability to penetrate tonsillar epithelial cells, the oral microbiota producing beta-lactamase in oropharynx area which protects GAS pathogens and reinfection may have important roles in the treatment failure of GAS infection by penicillin.21,22 However, as shown in the forest plot, antibiotic resistance of GAS to penicillin was low (4.2%) and not developed among isolated strains from Iranian children [Figure 3]. Similar results were reported in Germany (0%), Senegal (0%), Pakistan (0%), Argentina (0%), India (0%), and China (0%).23–28 In contrast, our results showed that GAS resistance to the rest of penicillins, including amoxicillin (38.3%), ampicillin (36.9%), carbenicillin (28.3%), and cloxacillin (79.0%) was high in Iran. A meta-analysis by Casey and Pichichero showed that oral cephalosporins’ therapeutic efficacy is better than oral penicillin.29 First-generation cephalosporins are used for GAS-induced pharyngitis therapy in penicillin-allergic patients (10 days).5 In the present meta-analysis, the prevalence of GAS strains resistant to cephalosporins was variable between 8.1% to 18.2%. To compare, the rate of cefotaxime-resistant GAS (8.8%) in Iran was higher than those reported from Germany (0%), Senegal (0%), Pakistan (1%), and India (0%),23–25,27 ceftriaxone-resistant GAS (8.1%) was higher than those of Senegal (0%) and Argentina (0%),24,26 and cefixime-resistant GAS (17.6%) was higher than that of Senegal (0%).24 Additionally, GAS resistance to narrow-spectrum cephalosporins, including cefazolin (10.7%), cephalexin (12.9%), and cephalothin (18.2%) was relatively low. Therefore, cephalexin can be used as an alternative treatment in penicillin-allergic patients. Other drugs that can be used in GAS-induced pharyngitis therapy in penicillin-allergic patients are oral macrolides.5 Despite the universal sensitivity of GAS strains to most antibiotics, the prevalence of macrolides-resistant strains are commonly found in some geographic areas.30 The prevalence of macrolide-resistant strains has reached 20% in several European countries, but it is still relatively low in the USA (5%).30 High macrolide resistance rates were reported in studies from Italy (31%) and Spain (26.6%), and low rates were reported in Turkey (4.8%), France (3.8%), and Sweden (3.7%).31 Target site modification and efflux pumps are two important mechanisms of GAS resistance to macrolides.31 The incidence of erythromycin- (5.4%), azithromycin- (12.0%), and clarithromycin-resistant (12.6%) S. pyogenes in Iran was low. On the other hand, GAS-induced pharyngitis treatment with tetracyclines is not recommended due to the high resistance.5 Similar findings were observed in this study, and the tetracycline resistance rate was high in Iran (30.4%), which is similar to other countries such as Senegal, India, China, South Korea, and Poland.24,27,28,32,33 The tet(M) and also rarely tet(O), tet(S), and tet(T) genes are associated with tetracycline resistance in S. pyogenes.3 Trimethoprim/sulfamethoxazole is also not recommended due to failure to eradicate bacteria from the pharynx.5 Our study indicated a high resistance rate of GAS strains (82.8%) to trimethoprim/sulfamethoxazole in Iran. Older fluoroquinolones (ciprofloxacin) and expensive and broad-spectrum newer fluoroquinolones are also not recommended for GAS-induced pharyngitis treatment.5 However, only 3.1% of GAS strains were resistant to ciprofloxacin and 11.9% to ofloxacin. In addition to macrolides, lincosamides (clindamycin and lincomycin) along with streptogramins are alternate options for GAS infections therapy in patients with allergy to penicillin or treatment failure.34 In Iran, 12.4% of GAS strains were resistant to clindamycin, while the low rate of resistance was found for clindamycin in studies from Senegal (0%), India (0%), Spain (0%), Japan (1.4%) and Germany (1.1%)24,27,35–37 as well as a high rate of resistance reported in studies from Pakistan (29%) and China (96.8%).25,28 Clindamycin can also be used to treat chronic pharyngeal GAS carriers, which occur in 20% of school-aged children during the winter and spring.5 Amoxicillin-clavulanic acid and rifampin are other effective drugs in eliminating GAS carriers.5 In this meta-analysis, 15.3% of GAS isolates were resistant to rifampicin and 89.5% to amoxicillin-clavulanic acid. It has also been reported that GAS resistance rate to aminoglycosides is rare worldwide.30 By contrast, in this study, 39.6% of GAS strains isolated from Iran were resistant to gentamicin, 57.6% to tobramycin, and 49.3% to kanamycin. Different factors can determine antibiotic efficacy. For example, misuse of antibiotics, self-medications, antibiotic concentration, host factors (serum effect), poor infection control in health care settings, poor hygiene, and bacterial status (biofilm, tolerance, and persistence) are the most important factors that have a great impact on antibiotic resistance prevalence.38
Conclusion
Rational and appropriate uses of antibiotics are important approaches in curbing the emergence of antibiotic-resistant isolates. The current meta-analysis indicated that GAS strains in Iran remained susceptible to commonly prescribed antibiotics to treat GAS infections, including penicillin and macrolides, lincosamides, and narrow-spectrum cephalosporins. Therefore, these drugs are the first-line treatments, respectively, among non-allergic and penicillin-allergic children in Iran. However, the GAS resistance rate to cefadroxil, which is recommended in penicillin-allergic patients, is unclear in Iran. We recommend that further studies to determine the major mechanisms of antibiotic resistance among GAS strains in Iran.
Disclosure
The authors declared no conflict of interests. No funding was received for this study.
references
- 1. Carroll KC, Butel JS, Morse SA. Jawetz Melnick & Adelbergs medical microbiology. 27th ed. Pennsylvania: McGraw Hill Professional; 2016, p. 215-220.
- 2. Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. 8th ed. UK: Elsevier Health Sciences; 2015, p. 186-192.
- 3. Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev 2014 Apr;27(2):264-301.
- 4. WHO. 2005. The current evidence for the burden of group A streptococcal diseases. WHO report. WHO, Geneva, Switzerland.
- 5. Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012 Nov;55(10):1279-1282.
- 6. Hasenbein ME, Warner JE, Lambert KG, Cole SE, Onderdonk AB, McAdam AJ. Detection of multiple macrolide- and lincosamide-resistant strains of Streptococcus pyogenes from patients in the Boston area. J Clin Microbiol 2004 Apr;42(4):1559-1563.
- 7. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009 Jul;6(7):e1000100.
- 8. Khosravi AD, Ebrahimifard N, Shamsizadeh A, Shoja S. Isolation of Streptococcus pyogenes from children with pharyngitis and emm type analysis. J Chin Med Assoc 2016 May;79(5):276-280.
- 9. Ghaffari M, Moaddab R, Rafi A, Roshdi Maleki M. Study of group A β-hemolytic streptococci colonization and resistance drug of isolates on 12-14 year old healthy students of aras free zone schools. Int J Med Microbiol 2013;6(4):40-50.
- 10. Nabipour F, Tayarzadeh MA. Beta hemolytic group A Streptococcal drug resistant to penicillin among asymptomatic carriers. Zahedan J Res Med Sci (TABIB-E-SHARGH) 2005; 7(2):131-137. [In Persian]
- 11. Kamaly A, Daneshy M, Kheirkah M. Sensitivity of penicillin in group A beta-hemolytic Streptococcus. J Qazvin Univ Med Sci 2001;5(1):68-72.
- 12. Mohsenimoghaddam F, Shahidizandi B, Shabani Shahrbabak Z, Rezaeian M, Jafarpour P, Hadavi M. Antibiotic resistance pattern on beta hemolytic (A) Streptococci among rafsanjan’s secondary school pupils in 2009. Majallah-i Ilmi-i Danishgah-i Ulum-i Pizishki-i Rafsanjan 2013;12(4):291-298.
- 13. Ardalan AA, Rad AF, Keshavarzi F. Antibiotic resistance and molecular analysis of Streptococcus pyogenes isolated from Iranian patients. Asian J Biol Sci 2014;7:284-293.
- 14. Kalantar EA, Sadighi V, Derakhshan S, Biranvand S, Torabi V. Determination of antibiotic resistance plane of group A Streptococci isolated from children with sore throat. J Microbiol Biotechnol 2011;3(8):7-12.
- 15. Sayyahfar S, Fahimzad A, Naddaf A, Tavassoli S. Antibiotic susceptibility evaluation of group A Streptococcus isolated from children with pharyngitis: a study from Iran. Infect Chemother 2015 Dec;47(4):225-230.
- 16. Parvizi E, Nateghian A, Ahmadi A, Mirsaeedi K, Irajian G. Antibiotic susceptibility of Streptococcus pyogenes isolated from throat cultures of healthy children aged between 5-15 years. Int J Mol Clin Microbiol 2014;2:411-416.
- 17. Jasir A, Tanna A, Noorani A, Mirsalehian A, Efstratiou A, Schalen C. High rate of tetracycline resistance in Streptococcus pyogenes in Iran: an epidemiological study. J Clin Microbiol 2000 Jun;38(6):2103-2107.
- 18. Nourouzi HR, Naderi Nasab M. The prevalence of pharyngeal carriers of group A beta-hemolytic Streptococcus and antibiotic susceptibility pattern of this bacteria in Zahedan, southeast of Iran. Iran J Otorhinolaryngol 2009;21(55):33-40.
- 19. Bordji a. Shahraki ZS, Naserpoor PT, Fazaeli A, Mohagheghi FA, Bocaiian M. Prevalence of beta hemolytic group A Streptococci among elementary school children in Zahedan, 2002. J Zanjan Univ Med Sci 2005;13(51):49-54.
- 20. Clinical and Laboratory Standards Institute. M100-S25 performance standards for antimicrobial susceptibility testing; Twenty-fifth informational supplement. CLSI. 2015; 35:1-240.
- 21. Brook I. Penicillin failure in the treatment of streptococcal pharyngo-tonsillitis. Curr Infect Dis Rep 2013 Jun;15(3):232-235.
- 22. Passàli D, Lauriello M, Passàli GC, Passàli FM, Bellussi L, Group A. Group A streptococcus and its antibiotic resistance. Acta Otorhinolaryngol Ital 2007 Feb;27(1):27-32.
- 23. Arvand M, Hoeck M, Hahn H, Wagner J. Antimicrobial resistance in Streptococcus pyogenes isolates in Berlin. J Antimicrob Chemother 2000 Oct;46(4):621-624.
- 24. Camara M, Dieng A, Boye CS. Antibiotic susceptibility of Streptococcus pyogenes isolated from respiratory tract infections in Dakar, Senegal. Microbiol Insights 2013;6: MBI-S12996.
- 25. Khan RM, Anwar S, Pirzada ZA. The emergence of lincosamide and macrolide resistance in Streptococcus pyogenes from Pakistan. Asian Biomed 2017;9(3):321-324.
- 26. Lopardo H. Antimicrobial resistance in ß-hemolytic Streptococci in Argentina. Communicating Research and Educational Topics and Trends in Applied Microbiology 2007:794-798.
- 27. Ray D, Saha S, Sinha S, Pal NK, Bhattacharya B. Molecular characterization and evaluation of the emerging antibiotic-resistant Streptococcus pyogenes from eastern India. BMC Infect Dis 2016 Dec;16(1):753.
- 28. Chang H, Shen X, Fu Z, Liu L, Shen Y, Liu X, et al. Antibiotic resistance and molecular analysis of Streptococcus pyogenes isolated from healthy schoolchildren in China. Scand J Infect Dis 2010;42(2):84-89.
- 29. Casey JR, Pichichero ME. Meta-analysis of cephalosporins versus penicillin for treatment of group A streptococcal tonsillopharyngitis in adults. Clin Infect Dis 2004 Jun;38(11):1526-1534.
- 30. Cattoir V. Mechanisms of antibiotic resistance. 2016.
- 31. Yamanaka N. Mechanisms of antibiotic resistance in Streptococcus pyogenes. Issues Infect Dis 2004;3:143-149.
- 32. Cha S, Lee H, Lee K, Hwang K, Bae S, Lee Y. The emergence of erythromycin-resistant Streptococcus pyogenes in Seoul, Korea. J Infect Chemother 2001 Jun;7(2):81-86.
- 33. Mlynarczyk G, Mlynarczyk A, Jeljaszewicz J. Epidemiological aspects of antibiotic resistance in respiratory pathogens. Int J Antimicrob Agents 2001 Dec;18(6):497-502.
- 34. Bisno AL, Gerber MA, Gwaltney JM Jr, Kaplan EL, Schwartz RH; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis 2002 Jul;35(2):113-125.
- 35. Rodríguez-Zurita ME, Solís del Baño S, Robres Guillén P, González Praetorius A, Gimeno Fernández C, Pérez Pomata MT, et al. [Susceptibility of Streptococcus pyogenes to macrolides and quinolones in Guadalajara, Spain]. Rev Esp Quimioter 2003 Mar;16(1):61-64.
- 36. Ikebe T, Hirasawa K, Suzuki R, Isobe J, Tanaka D, Katsukawa C, et al. Antimicrobial susceptibility survey of Streptococcus pyogenes isolated in Japan from patients with severe invasive group A streptococcal infections. Antimicrob Agents Chemother 2005 Feb;49(2):788-790.
- 37. Sauermann R, Gattringer R, Graninger W, Buxbaum A, Georgopoulos A. Phenotypes of macrolide resistance of group A streptococci isolated from outpatients in Bavaria and susceptibility to 16 antibiotics. J Antimicrob Chemother 2003 Jan;51(1):53-57.
- 38. Li J, Xie S, Ahmed S, Wang F, Gu Y, Zhang C, et al. Antimicrobial activity and resistance: influencing factors. Front Pharmacol 2017 Jun;8:364.