Acrodermatitis enteropathica (AE) is a rare autosomal recessive disorder that affects one in 500 000 children without the prediction of race and gender. It is caused by zinc malabsorption in the intestines. Symptoms differ according to age, but the main clinical manifestations include diarrhea, alopecia cutaneous lesions, and recurrent infections.1 Zinc levels in the serum and urine might help the diagnosis, but the definite diagnostic test is genetic testing of the gene mutation. Treatment is mainly by the administration of zinc therapy, which depends on the current level and body weight.2
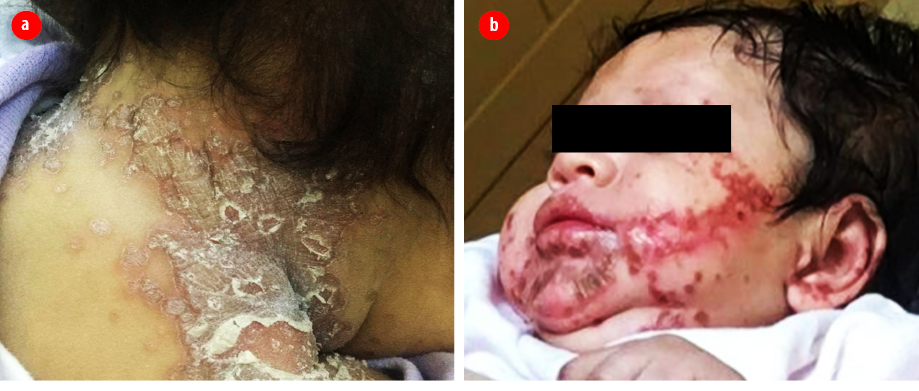
Figure 1: (a) Erythematous scaly lesions in the neck. (b) Perioral erythematous vesiculopustular lesions.
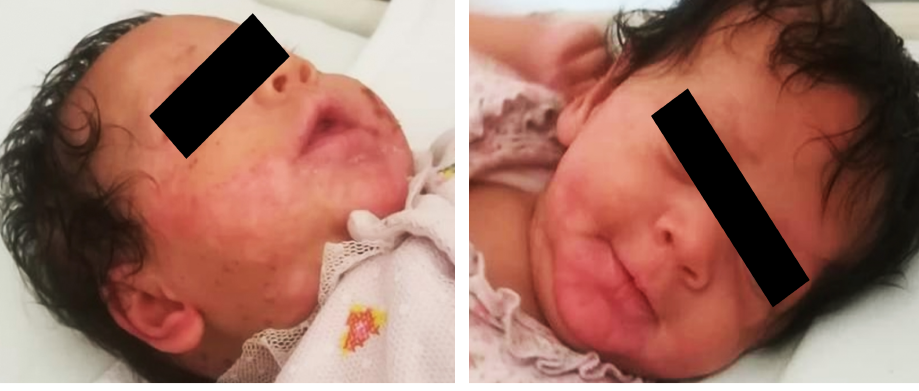
Figure 2: Remission of the skin lesions after one month of treatment.
Case report
We present a case of a two-month-old girl born at 33 weeks gestation by cesarean section due to severe maternal pre-eclampsia with a weight of 1.98 kg. She was admitted to the neonatal intensive care unit for three weeks and discharge, she remained healthy on breastfeeding. She was brought by her mother to the accident and emergency department with a one-month history of skin lesions that involved the perioral region, hands, and skin folds including napkin area. This was associated with cracked lips, hair loss, and diarrhea more than seven times per day. The mother noticed that her daughter was excessively crying, lethargic, and febrile. The patient was admitted previously to another hospital with superinfected skin lesions, which was treated with intravenous antibiotics for four days. At the initial assessment of the patient, she looked lethargic and dehydrated. Skin examination revealed erythematous scaly patches on the neck and nappy area as well as vesiculopustular lesions predominantly on the perioral region [Figure 1]. Alopecia was noticed as well. We considered the diagnosis of AE, and the patient was hospitalized. Laboratory tests were sent, which showed normal inflammatory markers, anti-tissue trans glutamine for celiac disease, β-lactoglobulin for cow’s milk protein intolerance, and immunoglobulins but low alkaline phosphatase (ALP) (45 IU/L). Zinc level was sent to a laboratory in France. While waiting for blood culture, urine cultures, and swabs from the lesions, the patient was started on intravenous cloxacillin, cefotaxime, and zinc therapy. Zinc therapy was started at a dose of 3 mg/kg daily of elemental zinc. During admission, the patient’s diarrhea stopped, and her skin lesions started to become paler and smaller after three to four days. All cultures and swabs did not reveal any growth. She completed 14 days of intravenous antibiotics and was discharged home to continue daily zinc therapy with a follow-up appointment at the pediatric dermatology clinic. The patient did not undergo genetic testing because of logistical reasons as it is only available in a single health care institution across the country.
At the follow-up appointment, we reported her zinc level as < 1.9 µmol/L. The patient improved, and complete remission of skin lesions and alopecia were noticed [Figure 2]. Her dose was adjusted to 0.5 mg/kg/day according to her weight. She was advised to continue with her zinc therapy and to continue regular follow-up visits with the pediatric gastroenterology and dermatology clinic at the regional hospital.
Discussion
AE is an autosomal recessive disorder, which is mainly caused by zinc deficiency (ZD). Zinc is an essential element in the body which has an important role in the human immune system, growth and development, hormones synthesis and activation, and gene regulation.3 It is also considered a co-enzyme for several enzymes, including ALP, alcohol dehydrogenase, RNA polymerase, and numerous digestive enzymes.4
ZD can be classified into inherited (genetic) and acquired. Patients at high risk of developing acquired ZD are those with malabsorption disorders such as celiac and Crohn’s disease, premature, low birth weight, and those who received exclusive parenteral low zinc breast milk feeding.5 Genetic ZD, also called AE, is mainly due to mutation in the SLC39A4 gene localized in 8q24.3, which encodes zinc/iron-regulated transporter-like protein 4.1,2 This protein facilitates zinc absorption in the intestine and its metabolism in the body.2 Our patient was born with a very low birth weight of 1.98 kg and was also premature, which put her at high risk of developing ZD.
The clinical presentation of AE differs according to the age group; however, the classical disease triad includes alopecia, periorificial dermatitis, and diarrhea. Our patient presented with diarrhea, which only accounts for 20% of all patients with AE. Infants usually present with diarrhea, neurological symptoms, and anorexia, while school children and toddlers experience growth retardation, alopecia, weight loss, and recurrent secondary infections.6 Severity of skin lesions might differ from an eczematous erythematous rash to vesicular to a bullous rash. Early recognition and treatment should be the primary goal to prevent further deterioration of the disease.7 In our patient, the rash was eczematous in the neck and napkin area while it appeared pustular in the perioral region. A previous study reported patients with only moist eczematous plaques distributed over the perioral and perineal region, as well as the buttocks.2
In our case, the patient was diagnosed promptly with AE because of her typical presentation with the classical symptoms and signs of perioral dermatitis, diarrhea, and alopecia. However, if the presentation is vague, other differential diagnoses, including atopic dermatitis, contact dermatitis, seborrheic dermatitis, psoriasis, and malabsorption disorders, should be considered.8 Serum zinc levels (normal value 12.2 to 18.2 µmol/L) is the most widely used test along with ALP (normal value 44 to 147 IU/L). In our patient, both serum zinc level and ALP were low, while in another study, ALP levels were normal.2 We were unable to get genetic testing for our patient. Access to genetic testing is only available in one institute and difficult to access by all patients all over the country.
AE requires lifelong follow-up, but the great thing about this disorder is that once it is diagnosed and treatment has been started, symptoms will disappear within few days, and the survival rate is 100%.2 Recommended elemental zinc therapy is 3 mg/kg/day as initial treatment followed by 1–2 mg/kg/day as a maintenance dose.9 Follow-up is required for adjustment of the dose according to the weight and to check for zinc toxicity as was done with our patient. Studies have shown that high zinc levels might lead to hypercupremia resulting in the alteration of the immune system and further complications.10
Conclusion
AE is a rare condition that requires timely recognition and management. Since our case presented with typical signs and symptoms, the diagnosis was made early and treatment was started accordingly. Follow-up is necessary to avoid zinc toxicity, adjust the dose according to the patient weight, and document the clinical progress to maintain the best quality of life.
Disclosure
The authors declared no conflicts of interest.
Acknowledgements
We would to thank the patient’s parents for their verbal consent and cooperation with us in completing this case report.
references
- 1. Wyllie R, Hyams JS. Pediatric gastrointestinal and liver disease e-book. Elsevier Health Sciences; 2010 Nov 29.
- 2. Kaur S, Sangwan A, Sahu P, Dayal S, Jain VK. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol 2016 Jan;17(1):35.
- 3. Costello RB, Grumstrup-Scott J. Zinc: what role might supplements play? J Am Diet Assoc 2000 Mar;100(3):371-375.
- 4. Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007 Jan;56(1):116-124.
- 5. Perafán-Riveros C, França LF, Alves AC, Sanches JA Jr. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol 2002 Sep-Oct;19(5):426-431.
- 6. Van Wouwe JP. Clinical and laboratory diagnosis of acrodermatitis enteropathica. Eur J Pediatr 1989 Oct;149(1):2-8.
- 7. Agarwal S, Gopal K. Acrodermatitis enteropathica in a breast-fed infant. Indian J Dermatol Venereol Leprol 2007 May-Jun;73(3):209.
- 8. Valdés R, Mauret M, Castro Á. [Acrodermatitis enteropathica: report of one case]. Rev Med Chil 2013 Nov;141(11):1480-1483.
- 9. Nistor N, Ciontu L, Frasinariu OE, Lupu VV, Ignat A, Streanga V. Acrodermatitis enteropathica: a case report. Medicine (Baltimore) 2016 May;95(20):e3553.
- 10. Shahsavari D, Ahmed Z, Karikkineth A, Williams R, Zigel C. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect 2014 Jul;4(3):24707.