|
Abstract
Objective: A prospective study was designed to analyze risk factors and clinical features in children with global developmental delay (GDD) at our hospital. No previous data is available on GDD from Oman.
Methods: This study was conducted at Sultan Qaboos University Hospital from January 2008 until June 2009. All the children aged 5 years or less, referred with suspected GDD were included in the study. Data was analyzed to determine the underlying etiology. The children with neurodegenerative disease and muscular dystrophy were excluded from the study.
Results: One hundred and ten children, 59 males (53.6%) and 51 females (46.4%) were included in the study. The mean age at initial evaluation was 13.29 months. An underlying etiology was determined in 79 (71.8%) children. Perinatal history was associated with significant difference in detection of etiology (p=0.039). Abnormal neurological examination was a significant factor in detection of the underlying etiology. Magnetic resonance imaging (MRI) in 105 children and metabolic screening in 93 children were the most frequently ordered investigations. Abnormal imaging, MRI (p=0.001), CT scan (p=0.036) and metabolic screening (p=0.034) were significantly associated with detection of etiology.
Conclusion: Etiology was detected in 71.8% of the children. MRI was the most significant investigation to detect the abnormality.
Keywords: Developmental delay; Children; Etiology; Asphyxia.
Introduction
Global developmental delay (GDD) is one of the most common reasons for referral to a pediatric neurologist.1 Global developmental delay is defined as performance that is two standard deviations or more below the mean on age-appropriate, standardized norm-referenced testing in at least two or more developmental fields of gross/fine motor, cognition, social/personal and activities of daily living.1-3 The term "global developmental delay" is usually reserved for younger children, typically less than 5 years of age.1 The prevalence of GDD is not precisely known, however, estimates of affected children range between 1% and 3%.1,4
Finding an underlying cause for a child's delay can help the neurologist in providing an estimation of the child's ultimate developmental potential and organize specific treatment requirement and intervention.1 In children with familial disorders, investigations could help the at-risk siblings. The reported yield of an underlying etiology is extremely variable ranging from 10% to 80%.1,5-8 This is mostly related to the criteria used and the extent of investigations performed at a particular center.
Although guidelines from North America and Europe were published, optimal approach for work up of a child with GDD remains unclear.3,9 On established guidelines, studies were conducted in North America and Turkey.1,10 There is also considerable non-uniformity with respect to the extent of investigations that should be undertaken.1 Not much data is available about the GDD in the Gulf or Arab countries. This study was conducted to determine the etiologic yield for GDD in children attending a tertiary care hospital in this part of the world.
Methods
The Department of Child Health (Neurology Unit) of Sultan Qaboos University Hospital is one of the highest tertiary centres in the country where neurology patients are referred from all over the country for evaluation and management. All the children referred to the department as GDD from January 2008 to June 2009 were assessed. They were included in the study if they fitted the definition of GDD.3 There was no exclusion of a particular group of children or etiology. The developmental assessment was based on well defined methods.11 Patients were excluded from the study if the age at presentation was over 5 years; if only one field of development was affected; and if the workup confirmed neurodegenerative disease or muscular dystrophy. The hospital ethical committee had approved the study.
Each child was assessed by a child neurologist. A thorough history was obtained in each child. Health card of each child was checked for antenatal, natal, and postnatal events. Selected laboratory investigations were done based on the suspected diagnosis.
Karyotyping and other genetic tests were obtained in patients with family history of GDD and dysmorphism. Metabolic tests on blood (tandem mass spectrometry, lactate, ammonia) and urine ketones were done in patients suspected to have metabolic disease. Wherever indicated, lysosomal tests were done to exclude neurodegenerative diseases of the brain.
Electrophysiological studies like electroencephalogram (EEG), visual-evoked potentials (VEP), and brainstem auditory evoked potential (BAEP) were done if the children had seizures, visual or hearing defects respectively. Serum creatine kinase was tested in children suspected to have muscle disease.
Perinatal asphyxia was diagnosed if the children presented with any of the following: the perinatal history was suggestive of fetal heart rate changes; meconium staining; or acidotic cord pH; subsequent neonatal encephalopathy; other organ or systemic dysfunction; and compatible electrophysiological tests and neuroimaging.1 Also, notes of the referring doctors from primary healthcare hospitals were looked into. In addition, perinatal history from parents were recorded. Neuroimaging (CT and or MRI brain) were performed in all patients to determine structural abnormality and rule out conditions like neurodegenerative diseases of the brain.
Parental consent was not required in the study as no invasive procedures were done. Further the work included routine tests only. No family refused the investigations. Severity of the developmental delay was categorized according to the overall functional age based on the results of developmental assessments to chronological age.5,10 Mild delay was defined if the functional age was 66% of chronological age, moderate delay as functional age 34% to 66% of chronological age, and severe delay as functional age below 33% of chronological age.1,10
All data was collected, charted and analyzed using SPSS Statistics 17.0 program. A p value of equal or below 0.05 was utilized as a significant difference in detection of etiologic diagnosis by each factor, clinical feature, and investigation.
Results
One hundred and ten children fulfilled the criteria of GDD, 59 (53.64%) were male and 51 (46.4%) were female, with male:female ratio of 1.16:1. The mean age at initial evaluation was 13.29 months (SD 10.69). Age when developmental delay was suspected was 5.83 months (SD: 4.32) and average interval between initial suspicion and initial evaluation was 7.46 months.
The etiology was detected in 79 children (71.8%) as shown in Table 1. The most common etiology was perinatal asphyxia (26 patients; 23.7%). Metabolic disorders were the next common cause seen in 13 patients (11.4%). Neuronal migration disorder or cerebral dysgenesis was seen in 12 patients (10.5%). Pachygyria and corpus callosum agenesis were the most common anomalies seen in four children each. There was one case of schizencephaly, polymicrogyria, Dandy-Walker anomaly, and multiple anomalies. Ten children with genetic syndromes were one each as a case of Bardet-Biedl syndrome, Prader-Willi syndrome, Angelman syndrome, Russell-Silver syndrome, Schwartz-Jampel syndrome, Smith-Lemli-Opitz syndrome and Cornelia de Lange syndrome. There were three with Joubert syndrome. Metabolic disorders seen were mitochondrial cytopathy, one each case of phenylketonuria, congenital lactic acidosis, 3-hydroxy butyric aciduria, 3-methyl glutaconic aciduria, and glutaric aciduria. Nonspecific white matter changes were seen in 15 patients (13.2%). Karyotyping was abnormal in two of 13 children investigated.
Table 1: Showing underlying etiology in GDD.
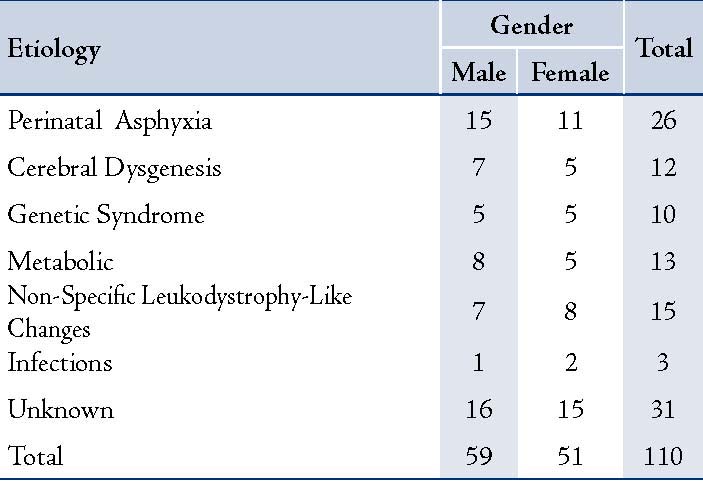
GDD of three children was attributed to CNS infections; one of them was due to neonatal meningitis, and two were due to congenital cytomegalovirus infection.
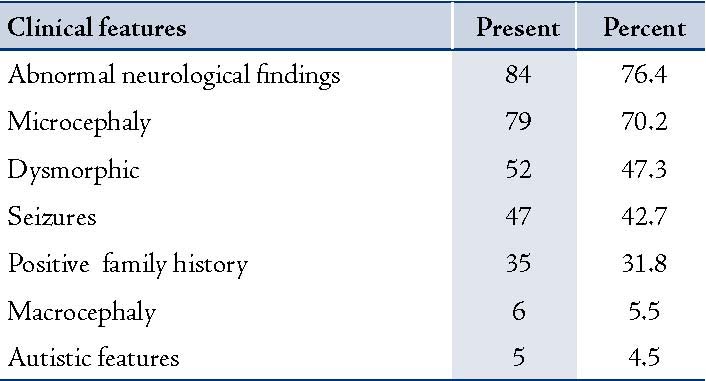
When different factors associated with GDD were analyzed, perinatal history had shown significant difference in the detection of etiology. Perinatal factors (risk factors) other than asphyxia associated with GDD were neonatal sepsis in 2, hyperbilirubinemia in 4 cases, hypoglycemia in 2 cases, seizures in 9 cases, prematurity in 7 and intrauterine growth retardation in 5 cases. Abnormal neurologic examination and microcephaly were the most common features. (Table 2)
Table 2: Showing other associated features with GDD
Medical imaging was performed in all cases. Out of 105 MRIs, 81.3% were abnormal, while CT brain was performed in 30 cases, 19 (63.3%) showed abnormality. The imaging abnormalities ranged from nonspecific white matter changes, age-related changes, mild volume loss to severe malformations called neuronal migration anomalies (cerebral dysgenesis), (Table 3). Metabolic screening was done in 93 (84.2%) and it was the second most common ordered investigation. Among the neurophysiology investigations, EEG was abnormal in 59.1%, (Table 3). The investigations with statistical significance in the detection of abnormality were MRI (p=0.001), metabolic screening (p=0.034), and CT (p=0.036). The GDD was mild in 15 cases, moderate in 51 cases, and severe in 44 children.
Table 3: Various laboratory investigation ordered and their Positive Yield.

Discussion
This study was hospital based and does not represent the community based study. There is possibility of bias towards working up of more affected children. The etiology was detected in 71.8% children with GDD, which was slightly higher but within the range reported from previous studies.5,6,8 Three previous studies from a single centre in North America had reported a yield of 63%,1 55%,5 and 38%.12 The first was a prospective study on 60 children and other two retrospective study on 80 and 261 children.1,5,12 In a retrospective study from Turkey on 247 patients with GDD, etiology was found in 63%.10 In a recent study from Jordan, the etiology was identified in 44.5% only.13 This study reported far less identifiable causes as compared to our study. Our yield is close to US and Turkish study.1,10 These differences in the yield are explained by the differences in the population, criteria used, and the extent of investigations. Another factor which has a major impact on detection of the underlying cause is the high incidence of consanguineous marriages resulting in familial cases in this part of the world.10
Perinatal asphyxia, metabolic diseases, cerebral dysgenesis, familial consanguinity, dysmorphism and non-specific white matter changes (leukopathy) in MRI constituted more than 87.2% of the etiology of GDD in the studied children. This distribution was similar to reports from previous studies.1,10,14 Perinatal asphyxia was the significant factor in detection of the etiology.
To reduce the incidence of familial conditions, premarital genetics checkup, and avoiding marriages in close relations are required. This needs national campaigns to increase the population awareness about the risks concerning consanguineous marriages. A pre-marriage genetic counseling should be encouraged especially if specific disorders are known to run in the family. As part of management of these children, this aspect was discussed with the parents. There are about 30 reports in different disorders regarding consanguinity from Oman. It has been reported to range from 56.3%15 to 70%16 depending on the disease entity. Neonatal screening should be adopted to detect metabolic disorders, especially since a large number of them have good developmental outcome if detected early and treatment is started early. In Oman, neonatal screening for metabolic disorders was recently started; however, screening for congenital hypothyroidism was previously established. This might explain why congenital hypothyroidism was not detected in the current studied population as a cause for GDD while metabolic disorders were seen.
Microcephaly was noted in about 70%, a significant number of the studied children. All had secondary microcephaly due to the underlying single or combination of factors affecting brain growth.
Association between dysmorphic features and GDD is variable. There are reports that dysmorphic features do not predict etiology.12,17 The conflict between these studies is probably related to the difference in the number of patients studied and also the subjective judgment about dysmorphic features. Dysmorphic features may, however, be predictive of genetic or chromosomal anomalies.1
Metabolic screening, considered a second line investigation in a previous study,9 was obtained in 84.5% of the studied children. It was positive in only 9.6% cases. It was significantly associated with the detection of etiologic diagnosis (p=0.034). This significance could be attributed to the lack of neonatal metabolic screening earlier with the high incidence of metabolic disorders in Oman. Metabolic diseases are common in this part of the world18; however, the neonatal screening for metabolic disorders has recently been started at SQUH.
The genetic syndromes were on lower side but within the range found in earlier studies. Chromosomal abnormalities were much lower and below the range.1,9,10 This could be due to non-referral of clear genetic cases to neurology as there was an established Genetic Clinic at the Ministry of Health.
Cerebral dysgenesis was an important etiology found in our children with GDD.19 MRI was the most frequently obtained investigation in the evaluation of GDD in our studied population. Neuroimaging studies had reported abnormalities in range of 9% to 80%.9,14 In our study, CT was positive in 62.5% while MRI was positive in 81.3%, similar to the above range.9,14 Both of these were significantly associated with identification of the etiology. The high yield of abnormal neuro-imaging has a direct correlation with abnormal neurological examination.1 Eighty four (76.4%) of our studied cases had abnormal neurological findings. This association was seen in previous studies.9,13,14 Nonspecific white matter changes not typical of known leukodystrophy marked as leukopathy as an etiologic cause for GDD were higher compared to previous reports.1 These can be as a result of unknown inherited disorders in the community yet to be discovered. All the blood tests including lysosomal enzymes were normal in these children.
Electrophysiological studies (EEG, VEP, and BAEP) were obtained quite frequently with relatively high-positive yield. None of them, however, were significantly associated with differences in the detection of etiology.
It is worth mentioning that psychosocial deprivation, toxin intake, endocrine causes, head trauma, and child abuse were looked into during this study; however, none were found. Such causes can be missed if the study is conducted over a short period. Occasional case of child abuse including shaken baby syndrome resulting in developmental delay are occasionally seen at our place but was not encountered during the study period.20
As reported earlier, an etiologic diagnosis for GDD can be determined in up to two thirds of the patients.1,10 Detection of an etiology helps in knowing the actual cause, accurate prognostication, and possible institution of specific therapeutic interventions.1
It is important to note that this study had limitations. Short time span of study being the main one. The spectrum of the yield probably would be wider if the study was conducted for a longer period, especially when dealing with very rare conditions.
Conclusion
The study gives a baseline data about the spectrum of GDD in the region. It also demonstrates that detection of an etiology for GDD is more likely in the presence of specific factors and clinical features. In addition, specific screening investigations should be obtained on the basis of clinical suspicion in order to improve the yield. With the recent establishment of Genetics Department at SQUH and Ministry of Health, efforts will be directed towards modification and prevention of familial genetic disorders to reduce the burden of these conditions in the families and community at large.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Srour M, Mazer B, Shevell MI. Analysis of clinical features predicting etiologic yield in the assessment of global developmental delay. Pediatrics 2006 Jul;118(1):139-145.
2. Fenichel GM. Psychomotor retardation and regression. In: Clinical pediatric neurology: a signs and symptoms approach, 4th ed. Philadelphia: WB Saunders, 2001;117–147.
3. Shevell MI, Ashwal S, Donley D, Flint J, Gingold M, Hirtz D, et al; Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology 2003 Feb;60(3):367-380.
4. Yeargin-Allsopp M, Murphy CC, Cordero JF, Decouflé P, Hollowell JG. Reported biomedical causes and associated medical conditions for mental retardation among 10-year-old children, metropolitan Atlanta, 1985 to 1987. Dev Med Child Neurol 1997 Mar;39(3):142-149.
5. Majnemer A, Shevell MI. Diagnostic yield of the neurologic assessment of the developmentally delayed child. J Pediatr 1995 Aug;127(2):193-199.
6. Shevell MI. The evaluation of the child with a global developmental delay. Semin Pediatr Neurol 1998 Mar;5(1):21-26.
7. Battaglia A, Bianchini E, Carey JC. Diagnostic yield of the comprehensive assessment of developmental delay/mental retardation in an institute of child neuropsychiatry. Am J Med Genet 1999 Jan;82(1):60-66.
8. Schaefer GB, Bodensteiner JB. Evaluation of the child with idiopathic mental retardation. Pediatr Clin North Am 1992 Aug;39(4):929-943.
9. McDonald L, Rennie A, Tolmie J, Galloway P, McWilliam R. Investigation of global developmental delay. Arch Dis Child 2006 Aug;91(8):701-705.
10. Ozmen M, Tatli B, Aydinli N, Calişkan M, Demirkol M, Kayserili H. Etiologic evaluation in 247 children with global developmental delay at Istanbul, Turkey. J Trop Pediatr 2005 Oct;51(5):310-313.
11. Egan DF, Illingworth RS, Mackeith RC. Developmental screening 0-5 years. William Heinemana Medical Books Ltd, 23 Bedford Square, WCI, London 1969.
12. Shevell MI, Majnemer A, Rosenbaum P, Abrahamowicz M. Etiologic determination of childhood developmental delay. Brain Dev 2001 Jul;23(4):228-235.
13. Masri A, Hammamy H, Khreisat A. Profile of developmental delay in children under five years of age in a highly consanguineous community: A hospital based study – Jordan. Brain and Development. 2010.
14. Shevell MI, Majnemer A, Rosenbaum P, Abrahamowicz M. Etiologic yield of subspecialists’ evaluation of young children with global developmental delay. J Pediatr 2000 May;136(5):593-598.
15. Rajab A, Patton MA. A study of consanguinity in the Sultanate of Oman. Ann Hum Biol 2000 May-Jun;27(3):321-326.
16. Khabori MA, Patton MA. Consanguinity and deafness in Omani children. Int J Audiol 2008 Jan;47(1):30-33.
17. Schaefer GB, Bodensteiner JB. Radiological findings in developmental delay. Semin Pediatr Neurol 1998 Mar;5(1):33-38.
18. Joshi SN, Hashim J, Venugopalan P. Pattern of inborn errors of metabolism in an Omani population of the Arabian Peninsula. Ann Trop Paediatr 2002 Mar;22(1):93-96.
19. Koul RL, Alfuitasi AM, Sankhla DK, Javad H, William RR. Pattern of childhood neuronal migrational disorders in Oman. Neurosciences (Riyadh) 2009 Apr;14(2):158-162.
20. Al-Saadoon M, Elnour IB, Ganesh A. Shaken baby syndrome as a form of abusive head trauma. Sultan Qaboos Univ Med J 2011 Aug;11(3):322-327.
|