Familial hypercholesterolemia (FH) is a common genetic disorder and if not recognized early and treated appropriately can lead to severe atherosclerosis and premature coronary artery disease (CHD).1,2
The prevalence of FH varies greatly between different studies depending on clinical criteria used and/or genetic confirmation.3,4 The most common clinical criteria used are the Dutch Lipid Clinic Network (DLCN)5 and the Simon Broome Registry criteria.2 These criteria are based on low-density lipoprotein cholesterol (LDL-C) levels of > 4.9 mmol/L (> 189.0 mg/dL), the presence of CHD in the patient or first-degree family member, and physical examination like tendon xanthoma or corneal arcus before the age of 40 years. FH is inherited as a monogenic mutation in the low-density lipoprotein receptor (LDLR), apolipoprotein-B (Apo B), or proprotein convertase subtilisin/kexin type 9 (PCSK9) genes.6 However, FH can also be inherited as a polygenic form from the contribution of several common LDL-C raising single nucleotide variants.7
Our study aimed to describe the clinical and genetic characteristics of FH in patients presented to the lipid clinic at Sultan Qaboos University Hospital (SQUH), Muscat, Oman.
Methods
SQUH is a tertiary hospital, and its lipid clinic is considered a referral center for FH patients across the country. In this study, a total of 450 patients who presented with high LDL-C (>189.0 mg/dL or 4.9 mmol/L) were recruited. The patients were stratified according to the DLCN criteria.5 As per the DLCN criteria, the diagnosis of FH was derived from scores from several criteria, including clinical and family history, physical examination, baseline cholesterol levels, and molecular testing. A ‘probable/definite’ FH was considered when the DLCN score was 6 or higher, and a ‘possible’ FH was made when the DLCN score was 3 to 5. Those with DLCN scores of < 3 were classified as ‘unlikely’ FH. Data collection included patients’ demographics, clinical history, physical examination, biochemical and radiological investigations as well as genetic analysis. The study was approved by the Sultan Qaboos University, College of Medicine and Health Science’s ethics committee (Reference: SQU-EC/172/18). All patients were required to sign informed consent forms.
Descriptive statistics were used to describe the data. For categorical variables, frequencies and percentages were reported. Differences between groups were analyzed using Pearson’s chi-squared tests (or Fisher’s exact tests for expected cells < 5). For continuous variables, mean and standard deviation were used to summarize the data while analysis was performed using ordinary least squares regression. An a priori two-tailed level of significance was set at the 0.05 level. Statistical analyses were conducted using STATA version 13.1 (STATA Corporation, College Station, TX, USA).
Results
The overall mean age of the cohort was 48.0±12.0 years, 56.0% (n = 252) were males and 11.3% (n = 51) were smokers. The proportion of patients with premature CHD, family history of FH, hypertension, and diabetes mellitus were 22.4% (n = 101), 99.6% (n = 448), 26.0% (n = 117), and 17.3% (n = 78), respectively. A total of 17.3% (n = 78) of the patients had percutaneous coronary intervention, 46.7% (n = 210) had high statin intensity therapy while 20.2% (n = 91) had statin and ezetimibe combination.
Table 1: Demographic and clinical characteristics of Oman familial hypercholesterolemia (FH) cohort stratified by the Dutch Lipid Clinic Network (DLCN) criteria.
|
Demographic |
|
|
|
|
|
|
Age, years, mean ± SD |
48.0 ± 12.0 |
41.0 ± 15.0 |
49.0 ± 12.0 |
44.0±13.0 |
< 0.001 |
|
Male gender |
252 (56.0) |
7 (63.6) |
177 (56.2) |
68 (54.8) |
0.847 |
|
Smoking |
51 (11.3) |
0 (0.0) |
45 (14.3) |
6 (4.8) |
0.039 |
|
Medical history |
|
|
|
|
|
|
History of FH |
448 (99.6) |
11 (100) |
314 (99.7) |
123 (99.2) |
0.510 |
|
Genetic testing |
72 (16.0) |
0 (0.0) |
15 (4.8) |
57 (46.0) |
< 0.001 |
|
Confirmed mutation* |
42 (58.3) |
0 (0.0) |
0 (0.0) |
42 (33.9) |
< 0.001 |
|
Tendon xanthomas |
31 (6.9) |
0 (0.0) |
0 (0.0) |
31 (25.0) |
< 0.001 |
|
Arcus corneal |
65 (14.4) |
1 (9.1) |
19 (6.0) |
45 (36.3) |
< 0.001 |
|
Diabetes mellitus |
78 (17.3) |
0 (0.0) |
57 (18.1) |
21 (16.9) |
0.432 |
|
Hypertension |
117 (26.0) |
1 (9.1) |
89 (28.3) |
27 (21.8) |
0.228 |
|
Hx of premature CAD |
101 (22.4) |
3 (27.3) |
59 (18.7) |
39 (31.5) |
0.041 |
|
Angina |
54 (12.0) |
1 (9.1) |
36 (11.4) |
17 (13.7) |
0.628 |
|
Myocardial infarction |
55 (12.2) |
2 (18.2) |
35 (11.1) |
18 (14.5) |
0.650 |
|
Hx of premature CBVD |
14 (3.1) |
1 (9.1) |
8 (2.5) |
5 (4.0) |
0.428 |
|
Hx of premature PAD |
3 (0.7) |
0 (0.0) |
2 (0.6) |
1 (0.8) |
0.479 |
|
Procedures and investigations |
|
|
|
|
|
|
PCI |
78 (17.3) |
2 (18.2) |
48 (15.2) |
28 (22.6) |
0.229 |
|
CABG |
21 (4.7) |
0 (.0.) |
8 (2.5) |
13 (10.5) |
0.008 |
|
Hx of CT angiogram |
30 (6.7) |
1 (9.1) |
13 (4.1) |
16 (12.9) |
0.012 |
|
Hx of CT coronary calcium score |
35 (7.8) |
0 (0.0) |
16 (5.1) |
19 (15.3) |
0.007 |
|
Hx of echocardiography, |
91 (20.2) |
1 (9.1) |
66 (21.0) |
24 (19.4) |
0.881 |
|
Hx of carotid doppler |
3 (0.7) |
0 (0.0) |
0 (0.0) |
3 (2.4) |
0.086 |
|
Statin therapy** |
|
|
|
|
|
|
Low-intensity statin therapy |
56 (12.4) |
3 (27.3) |
49 (15.6) |
4 (3.2) |
|
|
Medium-intensity statin therapy |
184 (40.9) |
2 (18.2) |
156 (49.5) |
26 (21.0) |
< 0.001 |
|
High-intensity statin therapy |
210 (46.7) |
6 (54.5) |
110 (34.9) |
94 (75.8) |
|
SD: standard deviation; Hx: history; CAD: coronary artery disease; CBVD: cerebrovascular disease; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; CT: computed tomography.
Percentages might not add to 100% due to rounding off.
*Out of the 16.0% (72/450) that had genetic testing, 58.3% (42/72) had low-density lipoprotein receptor mutation.
**High-intensity statin therapy was defined as those on atorvastatin 40–80 mg and rosuvastatin 20–40 mg, while medium-intensity statin therapy was defined as those on atorvastatin 10–20 mg, rosuvastatin 5–10 mg, simvastatin 20–40 mg, and pravastatin 40–80 mg. There were no patients on fluvastatin or lovastatin.
Table 2: Statin therapy stratified by atherosclerotic vascular disease (ASCVD) risk.
|
Low-intensity statin therapy |
56 (12.4) |
9 (6.1) |
47 (15.6) |
0.006 |
|
Medium-intensity statin therapy |
184 (40.9) |
58 (39.2) |
126 (41.7) |
|
High-intensity statin therapy |
210 (46.7) |
81 (54.7) |
129 (42.7) |
*High-intensity statin therapy was defined as those patients on atorvastatin 40–80 mg and rosuvastatin 20–40 mg. Medium-intensity statin therapy was defined as those on atorvastatin 10–20 mg, rosuvastatin 5–10 mg, simvastatin 20–40 mg and pravastatin 40–80 mg. There were no patients on fluvastatin or lovastatin.
Table 3: Lipid profiles of Omani familial hypercholesterolemia cohort stratified by the Dutch Lipid Clinic Network (DLCN) criteria.
|
Total cholesterol |
|
Baseline |
8.0 ± 1.4 |
6.8 ± 0.1 |
7.6 ± 0.8 |
9.2 ± 1.9 |
< 0.001 |
|
Post-index |
5.3 ± 1.4 |
4.2 ± 1.0 |
5.1 ± 1.2 |
5.8 ± 1.8 |
< 0.001 |
|
LDL-cholesterol |
|
Baseline |
6.1 ± 1.3 |
4.9 ± 0.0 |
5.7 ± 0.7 |
7.4 ± 1.8 |
< 0.001 |
Data are given as mean ± standard deviation.
LDL: low-density lipoprotein.
Table 4: Lipid profiles of Omani familial hypercholesterolemia patients stratified by the atherosclerotic vascular disease risk status.
|
Total cholesterol |
|
|
|
|
All (n = 329) |
8.1 ± 1.4 |
5.4 ± 1.5 |
< 0.001 |
|
High risk (n = 216) |
8.1 ± 1.3 |
5.4 ± 1.3 |
< 0.001 |
|
Very high risk (n = 113) |
8.2 ± 1.6 |
5.3 ± 1.7 |
< 0.001 |
|
LDL-cholesterol |
|
|
|
|
All (n = 331) |
6.3 ± 1.4 |
3.4 ± 1.3 |
< 0.001 |
|
High risk (n = 217) |
6.2 ± 1.3 |
3.5 ± 1.3 |
< 0.001 |
|
Very high risk (n = 114) |
6.3 ± 1.6 |
3.3 ± 1.3 |
< 0.001 |
|
Non-HDL-cholesterol |
|
|
|
|
All (n = 320) |
6.9 ± 1.4 |
4.1 ± 1.5 |
< 0.001 |
|
High risk (n = 213) |
6.9 ± 1.4 |
4.1 ± 1.4 |
< 0.001 |
SD: standard deviation; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
At baseline, 415 patients had populated lipid profiles while at follow-up 366 patients had their lipid levels documented.
Table 1 shows demographic, clinical, and statin characteristics of the patients according to DLCN criteria. The proportion of ‘probable/definite’, ‘possible’, and ‘unlikely’ FH were 27.6% (n = 124), 70.0% (n = 315), and 2.4% (n = 11), respectively. Compared to the ‘unlikely’ FH cohort, the ‘probable/definite’ FH group more likely to be associated with confirmed mutations (33.9% vs. 0.0; p < 0.001), tendon xanthomas (25.0% vs 0.0; p < 0.001), arcus corneal (36.3% vs 9.1%; p < 0.001), coronary artery bypass graft (10.5% vs. 0.0; p = 0.008), high-intensity statin therapy (75.8% vs. 54.5%; p < 0.001), and statin ezetimibe combination (50.8% vs. 27.3%; p < 0.001). Furthermore, those with very high atherosclerotic vascular disease (ASCVD) risk were also associated with high-intensity statin therapy (55% vs. 43%; p = 0.006) and statin ezetimibe combination (26% vs. 17%; p = 0.023) [Table 2].
Lipid profiles of Omani FH patients stratified by the DLCN criteria are outlined in Table 3. All lipid fractions (total cholesterol (TC) and LDL-C) at baseline and post-index were significantly higher in the ‘probable/definite’ FH group compared to the ‘unlikely’ FH cohort (all p-values < 0.001). When compared to the baseline period [Table 4], post-index lipid levels that included TC (5.4 vs. 8.1 mmol/L; p < 0.001), LDL-C (3.5 vs. 6.2 mmol/L; p < 0.001), and non high-density lipoprotein cholesterol (non HDL-C) (4.1 vs. 6.9 mmol/L; p < 0.001) were significantly lower in not only the high ASCVD risk but also very high ASCVD risk TC (5.3 vs. 8.2 mmol/L; p < 0.001), LDL-C (3.3 vs. 6.3 mmol/L; p < 0.001), and non HDL-C (4.2 vs. 7.0 mmol/L; p < 0.001).
Figure 1 represents LDL-C goal attainment at admission stratified by the DLCN and ASCVD criteria, respectively. Patients with ‘probable/definite’ FH were less likely to achieve their LDL-C goal attainment compared to those with ‘unlikely’ FH (13.0% vs. 57.1%; p < 0.001). Those with very high ASCVD risk were less likely to their LDL-C goals compared to the high ASCVD risk cohort (9.6% vs. 32.0%; p < 0.001).
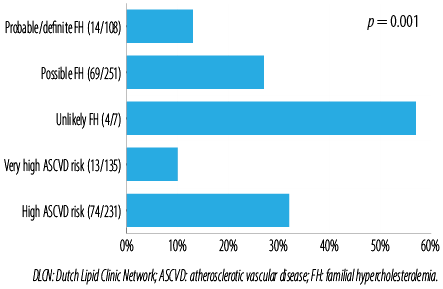
Figure 1: Low-density lipoprotein cholesterol goal attainment of the cohort stratified by DLNC and ASCVD risk.
Discussion
This paper describes the clinical and the genetic characteristics of patients referred to the lipid clinic at SQUH as a suspected case of FH. Patients were stratified according to the DLCN, with 27.6% patients diagnosed as ‘probable/definite’ FH, 70.0% as ‘possible’ FH, and 2.4% as the ‘unlikely’ FH group. There are few case reports about FH and its genetic characteristics in Oman.8–13 Currently, most suspected FH cases are referred to SQUH lipid clinic for genetic confirmation and advanced management.
FH remains underdiagnosed worldwide.14 There are no wide national registries for FH in Oman. The “Oman cascade screening to prevent early onset risk of cardiovascular diseases in FH Young children and adult index cases and their families (OMANORYX) study”15 is an ongoing study aimed to initiate early detection and treatment of FH in Oman through detection of index cases and cascade screening of first- and second-degree relatives to prevent CVD outcomes. The Gulf Familial Hypercholesterolemia Registry (Gulf FH)8 was a cross-sectional and prospective study to determine the FH prevalence, genetic characteristics, and current management in the adult patients living in the Arabian Gulf region. The prevalence of FH (based on both probable and possible FH) was 0.96%, 1/104 (332/34 366). In Oman, the expected number of FH patients according to the Gulf FH registry9 prevalence of 1/104 and the Omani population of 2 994 601 (based on the 2019 census16) is around 28 794 patients. This indicates a significant gap in the diagnosis of FH in Oman, and the OMANORYX study may help to decrease this gap. Nonetheless, there is a definite need to engage local health care authorities to establish quality improvement programs, policies, and centers like lipid clinics for FH screening, diagnosis, and management.15
FH remains undertreated worldwide.9,14,17 In the SAFEHEART Registry,17 only 11% of the FH patients reached their target goal of < 2.6 mmol/L despite being on maximum lipid-lowering treatments. In our study, the overall goal attainment of LDL-C < 2.6 mmol/L was achieved in 24.0% (data is not shown) of patients while only 10.0% of FH patients with very high risk and LDL-C goal of < 1.8 mmol/L was achieved. High-intensity statins were prescribed in only 46.7% of patients, and 20.2% of patients were on both statins and ezetimibe.
Lipoprotein apheresis10 is only available at SQUH in Oman, and patients across the country are being referred to our center for the treatment of homozygous and severe heterozygous FH patients who do not respond to the maximum tolerated lipid-lowering treatments.
The Oman Society for Lipid and Atherosclerosis with support from the International Atherosclerosis Society has established an educational program like the ‘Lipid Metabolism and Cardiovascular Risk’ and ‘Severe FH’ courses18 to improve the knowledge and the management of various lipid disorders and FH in the Middle East and North Africa (MENA) region. Moreover, there are consensus clinical recommendations for the management of FH in the region.19,20
Although molecular testing of FH is considered as a confirmatory step in the diagnosis and important to screen the family members of the index cases, it is still not widely available worldwide, and few centers perform them in the MENA region.21 In Oman, molecular testing for FH is performed in the biochemistry laboratory at SQUH.11–13 The genetic test is only performed in 16.0% of suspected cases due to limited resources. As expected, the mutation was confirmed in the ‘probable/definitive’ FH group (33.9%), and the most common type of mutation was the LDLR mutation. A total of 58.3% of the suspected cases were reported to have causative FH mutation. The rest of the cases were more likely to have a polygenic form of hypercholesterolemia.
Conclusion
FH in Oman is still underdiagnosed and undertreated. Despite the use of high-intensity statin and combination therapies, a significant number of high and very ASCVD FH patients did not reach the LDL-C therapeutic goals. This implies the high need to engage the local health care authorities to set recommendations and quality improvement programs and policies for FH screening, diagnosis, and aggressive management.
Disclosure
The authors declared no conflicts of interest. This study received funding from Sultan Qaboos University, His Majesty’s Trust Fund Grant project code of SR/MED/BIOC/18/01.
references
- 1. Goldstein JK, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds), The Metabolic & Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. p. 2863-2913.
- 2. Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol 2004 Sep;160(5):407-420.
- 3. Henneman L, McBride CM, Cornel MC, Duquette D, Qureshi N. Screening for familial hypercholesterolemia in children: what can we learn from adult screening programs?. Healthcare (Basel) 2015 Oct;3(4):1018-1030.
- 4. De Backer G, Besseling J, Chapman J, Hovingh GK, Kastelein JJ, Kotseva K, et al; EUROASPIRE Investigators. Prevalence and management of familial hypercholesterolaemia in coronary patients: An analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis 2015 Jul;241(1):169-175.
- 5. Civeira F; International panel on management of familial hypercholesterolemia. Guidelines for the diagnosis and management of heterozygous familial hypercholesterolemia. Atherosclerosis 2004 Mar;173(1):55-68.
- 6. Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest 2003 Jun;111(12):1795-1803.
- 7. Trinder M, Francis GA, Brunham LR. Association of Monogenic vs Polygenic Hypercholesterolemia With Risk of Atherosclerotic Cardiovascular Disease. JAMA Cardiol 2020 Feb;5:390-399.
- 8. Al-Rasadi K, Alhabib KF, Al-Allaf F, Al-Waili K, Al-Zakwani I, AlSarraf A, et al. The gulf familial hypercholesterolemia registry (Gulf FH): design, rationale and preliminary results. Curr Vasc Pharmacol 2020;18(1):57-64.
- 9. Blom DJ, Almahmeed W, Al-Rasadi K, Azuri J, Daclin V, Kayikcioglu M, et al; ICLPS study group. Low-density lipoprotein cholesterol goal achievement in patients with familial hypercholesterolemia in countries outside Western Europe: The International ChoLesterol management Practice Study. J Clin Lipidol 2019 Jul - Aug;13(4):594-600.
- 10. Al-Dughaishi T, Al-Waili K, Banerjee Y, Sheik S, Al-Sabti H, Al-Zakwani I, et al. Successful direct adsorption of lipoproteins (DALI) apheresis during pregnancy in an Omani woman with homozygous familial hypercholesterolemia. Open Cardiovasc Med J 2015 Dec;9:114-117.
- 11. Al-Rasadi K, Al-Waili K, Al-Zidi WA, Al-Abri AR, Al-Hinai AT, Al-Sabti HA, et al. Low-density lipoprotein receptor gene mutation analysis and structure-function correlation in an Omani arab family with familial hypercholesterolemia. Angiology 2014 Nov;65(10):911-918.
- 12. Al-Waili K, Al-Zidi WA, Al-Abri AR, Al-Rasadi K, Al-Sabti HA, Shah K, et al. Mutation in the PCSK9 gene in Omani Arab subjects with autosomal dominant hypercholesterolemia and its effect on PCSK9 protein structure. Oman Med J 2013 Jan;28(1):48-52.
- 13. Al-Hinai AT, Al-Abri A, Al-Dhuhli H, Al-Waili K, Al-Sabti H, Al-Yaarubi S, et al. First case report of familial hypercholesterolemia in an Omani family due to novel mutation in the low-density lipoprotein receptor gene. Angiology 2013 May;64(4):287-292.
- 14. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al; European atherosclerosis society consensus panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013 Dec;34(45):3478-90a.
- 15. Wilemon KA, Patel J, Aguilar-Salinas C, Ahmed CD, Alkhnifsawi M, Almahmeed W, et al; Representatives of the global familial hypercholesterolemia community. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol 2020 Jan;5(2):217-229.
- 16. Sultanate of Oman. Ministry of National Economy. Archived from the original on 2010-11-13. Retrieved 2010-11-13. [cited 2020 April 20]. Available from: https://wayback.archive-it.org/all/20101113142325/http://www.moneoman.gov.om/%2fDefault-ar.aspx.
- 17. Perez de Isla L, Alonso R, Watts GF, Mata N, Saltijeral Cerezo A, Muñiz O, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatments goals in patients with familial hypercholesterolemia. 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016 Mar;67(11):1278-1285.
- 18. Member Society Activities. [cited 2020 April 20]. Available from: https://www.athero.org/programs-activities/member-society-programs/.
- 19. Al Rasadi K, Almahmeed W, AlHabib KF, Abifadel M, Farhan HA, AlSifri S, et al. Dyslipidaemia in the middle east: current status and a call for action. Atherosclerosis 2016 Sep;252:182-187.
- 20. Al-Ashwal A, Alnouri F, Sabbour H, Al-Mahfouz A, Al-Sayed N, Razzaghy-Azar M, et al. Identification and treatment of patients with homozygous familial hypercholesterolaemia: information and recommendations from a middle east advisory panel. Curr Vasc Pharmacol 2015;13(6):759-770.
- 21. Bamimore MA, Zaid A, Banerjee Y, Al-Sarraf A, Abifadel M, Seidah NG, et al. Familial hypercholesterolemia mutations in the Middle Eastern and North African region: a need for a national registry. J Clin Lipidol 2015 Mar-Apr;9(2):187-194.