Endobronchial ultrasound-guided trans-bronchial needle aspiration (EBUS-TBNA) is a modality with remarkable diagnostic yield and is less invasive than video-assisted thoracoscopic surgery or transthoracic needle aspiration.1,2
During the last decade, EBUS-TBNA has attained widespread acceptance as a minimally invasive and accurate technique for investigating mediastinal lymph nodes (LNs) to diagnose both non-malignant and malignant diseases.3 In cases with suspected malignancy, EBUS has been used for diagnosing and staging in patients with lung cancer.2,4–8
Real-time visualization of the LNs during sampling provides useful information about its structural and morphological characteristics.9 There is increasing interest in the sonographic features of the mediastinal LNs, which are consistent with pathology results.9–11 Definite sonographic nodal features, including increased size, distinct margin, round shape, heterogeneous echogenicity, increased color Dopper flow of the nodes, and signs of coagulation necrosis are more commonly observed within malignant nodes versus non-malignant ones.5,12
In this study, which was conducted for the first time among the Iranian population, the utility of the ultrasonographic morphological characteristics in patients undergoing EBUS was used to distinguish non-malignant from malignant LNs.
Methods
Individuals who underwent EBUS-TBNA due to hilar or mediastinal lymphadenopathy with an unknown cause or lung cancer stage at the interventional bronchoscopy unit of the Masih Daneshvari Hospital from January 2017 to January 2018 were enrolled in this study.
Contrast-enhanced chest computed tomography (CT) or positron emission tomography-CT as the conventional diagnostic tools coupled with other relevant investigations such as serum angiotensin-converting enzyme levels and tuberculin skin tests had been performed before the procedure.
Informed written consent was attained from all patients or their parents in the case of minors. The research was approved through the ethics committee of the National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences (IR.SBMU.NRITLD.REC.1396.291). No complications were seen with EBUS-TBNA.
All procedures were performed using FUJINON CP-8000 (Fujifilm Corporation, Minato-Ku, Tokyo, Japan) under general anesthesia by the endotracheal tube rigid bronchoscopy. Under standard monitoring of heart rate, blood pressure, and pulse oximetric saturation (O2 sat), conscious sedation was induced using a combination of intravenous fentanyl sodium and midazolam.
The endoscopic ultrasound scanner (EB530US, Fujifilm, Tokyo, Japan) was used to process the ultrasound imaging. The frequency was set at 10 MHz. LNs digital and video images were investigated by experienced sonographers (masked from the EBUS-TBNA results).
The international TNM staging system (reported in the literature) was used to determine LNs stations and numbers.13,14 Images were obtained using the probe, directly. The ultrasonographic features of each LN were determined and recorded before the procedure.
All LNs were categorized by the following characteristics: (1) size: based on the long and short axis; (2) shape: round if the ratio of the short axis to the long axis was < 1.5 or oval whenever the ratio was > 1.5 or triangular when the short and long axis had two perpendicular directions; (3) margin: indistinct (unclear margin) or distinct (if > 50% of the margin was visible); (4) central hilar structure (CHS): existence or absence; (5) echogenicity: homogeneous (uniform echo pattern) or heterogeneous (do not show uniform echo pattern); (6) presence or absence of calcification; (7) vascular patterns: avascular, hilar perfusion, and non-hilar perfusion; and (8) presence or absence of coagulation necrosis signs: hypoechoic area in the LNs without blood flow.15
Each LN was evaluated with power Doppler followed by color Doppler. At least three aspirates were obtained from each LN station. N3 nodes were sampled first, and then sampling proceeded to N2 and N1, respectively. TBNA LNs sampling was conducted using a 22-gauge needle. The pressure of suction was kept between 15 and 20 mL. The specimens were put into formalin containers and sent for pathological diagnosis. The pathologist was masked from the sonographic features of LNs.
Since Iran is an endemic area for tuberculosis, all specimens were sent for acid-fast staining as well as mycobacterial cultures and gene-Xpert MTB-RIF investigation. No further tissue confirmation was requested if EBUS-TBNA results were positive for malignancy. Immunohistochemistry was conducted in several cases. In each case, where an unequivocal malignancy presence was seen, a-six-month clinical and radiological follow-up was conducted. The final diagnosis was based on the microscopic examination of TBNA specimens by a pathologist. The pathology reports of the patients were collected and recorded.
Finally, the acceptable samples were classified into non-malignant and malignant groups. All features of LNs were compared with the pathological results as ‘non-malignant’ or ‘malignant’ using SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). The qualitative characteristics of non-malignant and malignant nodes were measured by chi-square Fisher’s exact test, and the quantitative characteristics were investigated through t-test. A p-value < 0.050 was considered significant. Significant variables of those which were important from a clinical point of view were entered into a multivariable logistic regression model. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated to differentiate between the malignant and non-malignant LNs.
Table 1: Baseline specifications of individuals and lymph nodes (LNs).
|
Number of individuals, (%) |
28 (30.1) |
65 (69.9) |
|
Age, mean ± SD, years |
37.3 ± 13.0 |
45.0 ± 14.2* |
|
Gender, male/female, (%) |
20 (36.4)/
8 (21.1) |
35 (63.6)/
30 (78.9) |
|
LN size, mean ± SD, cm |
2.1 ± 0.5 |
1.4 ± 0.5* |
|
LN stations included in the study |
|
4R |
4 |
16 |
|
4L |
1 |
5 |
|
7 |
36 |
80 |
|
10R |
3 |
0 |
|
10L |
4 |
4 |
|
11L |
21 |
38 |
|
11R |
5 |
23 |
|
12L |
0 |
11 |
|
12R |
0 |
2 |
*Significant p < 0.050; SD: standard deviation.
Results
In the current clinical trial, 93 patients who underwent EBUS-TBNA were studied in the interventional unit of the hospital. Baseline characteristics of patients and LNs based on pathology results are represented in Table 1.
The final diagnoses of patients are represented in Figure 1. Twenty-eight cases (74 LNs) were diagnosed as malignant, and 65 cases (179 LNs) were non-malignant. Of 65 non-malignant cases, 24 were granulomatous, 12 were anthracosis, and 29 were nondiagnostic (104 LNs). Therefore, the reactive group were followed-up for six months. During those six months, they did not show any signs or symptoms of malignancy and were considered reactive.
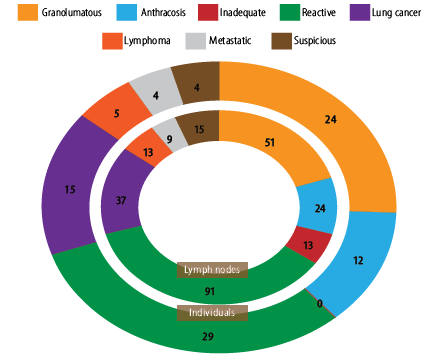
Figure 1: Final diagnosis result (patients = 93; lymph nodes = 253).
Eighty-six samples of the diagnostic specimens were obtained using suction, and 87 diagnostic samples were taken without suction. No significant diagnostic difference was seen between the samples collected with and without suction (p = 0.282).
As shown in Table 2, the differences between the characteristics of non-malignant and malignant nodes were statistically significant.
Factors that were clinically important were entered into a multivariable logistic regression model and the results showed five independent predictive factors for malignancy [Table 3] including size > 1 cm, heterogeneous echogenicity, hyperechogenicity, the existence of necrosis signs, and the absence of CHS. Of all the malignant LNs, 100% had at least one of these independent factors.
The accuracy of independent factors, including size > 1 cm, heterogeneous echogenicity, hyperechogenicity, the presence of necrosis signs, and absence of CHS were 42.3%, 71.5%, 71.9%, 73.5% and, 65.6%, respectively [Table 4].
Some representative morphological findings of EBUS characteristics, including CHS, shape, coagulation necrosis signs, calcification, margin, echogenicity, and vascular pattern, are presented in Figure 2.
Table 2: The characteristics of non-malignant and malignant nodes.
|
Shape |
|
|
|
|
|
|
Round |
40 |
22.3 |
57 |
77.0 |
< 0.001‡* |
|
Oval |
128 |
71.5 |
14 |
18.9 |
|
Triangular |
11 |
6.1 |
3 |
4.1 |
|
Vascular pattern |
|
|
|
|
|
|
Avascular |
48 |
26.8 |
6 |
8.1 |
< 0.001†* |
|
Hilar |
98 |
54.7 |
20 |
27.0 |
|
Non-hilar |
33 |
18.4 |
48 |
64.9 |
|
Echogenicity |
|
|
|
|
|
|
Homogenous |
124 |
69.3 |
17 |
23.0 |
< 0.001†* |
|
Heterogeneous |
55 |
30.7 |
57 |
77.0 |
|
Hypo |
130 |
72.6 |
22 |
29.7 |
< 0.000†* |
|
Hyper |
49 |
27.4 |
52 |
70.3 |
|
Margin |
|
|
|
|
|
|
Indistinct |
95 |
53.1 |
20 |
27.0 |
< 0.001†* |
|
Distinct |
84 |
46.9 |
54 |
73.0 |
|
Coagulation necrosis sign |
|
|
|
|
|
|
Absence |
137 |
76.5 |
25 |
33.8 |
< 0.001†* |
|
Presence |
42 |
23.5 |
49 |
66.2 |
|
Calcification |
|
|
|
|
|
|
Absence |
152 |
84.9 |
74 |
100.0 |
< 0.001‡* |
|
Presence |
27 |
15.1 |
0 |
0.0 |
|
Central hilar structure |
|
|
|
|
|
|
Absent |
73 |
40.8 |
60 |
81.1 |
*Significant p < 0.050.; †Chi-square; ‡Fisher’s exact.
Table 3: Adjusted and crude analyses of endobronchial ultrasonography image variables for predicting malignancy.
|
Shape |
|
|
|
|
|
Round |
0.016* |
5.225 (1.369–19.936) |
0.861 |
1.188 (0.174–8.110) |
|
Vascular pattern |
|
|
|
|
|
Non-hilar |
< 0.001* |
7.127 (3.706–13.707) |
0.301 |
1.791 (0.594–5.397) |
|
Echogenicity |
|
|
|
|
|
Heterogeneous |
< 0.001* |
7.559 (4.035–14.163) |
< 0.001* |
6.387 (2.426–16.817) |
|
Hyper |
< 0.001* |
6.271 (3.452–11.392) |
0.034* |
3.100 (1.088–8.834) |
|
Margin |
|
|
|
|
|
Distinct |
< 0.001* |
3.054 (1.691–5.515) |
0.485 |
1.432 (0.523–3.923) |
|
Coagulation necrosis sign |
|
|
|
|
|
Presence |
< 0.001* |
6.393 (3.534–11.567) |
0.013* |
3.527 (1.310–9.496) |
|
Size > 1 cm |
0.005* |
4.560 (1.563–13.301) |
0.050* |
4.687 (1.002–21.919) |
|
Absence of calcification |
0.998 |
0.000 |
0.998 |
0.000 |
* Significant p < 0.050. CHS: central hilar structure; CI: confidence interval; OR: odds ratio.
Table 4: Predictive value of each sonographic feature of EBUS for malignancy.
|
Size > 1 cm |
94.6 |
20.7 |
33.0 |
90.2 |
42.3 |
|
Round shape |
77.0 |
77.7 |
58.8 |
89.1 |
77.5 |
|
Non-hilar vascular pattern |
64.9 |
81.8 |
59.3 |
85.1 |
77.5 |
|
Distinct margin |
73.0 |
53.1 |
40.0 |
83.0 |
58.9 |
|
Echogenicity |
|
|
|
|
|
|
Heterogeneous |
77.0 |
69.3 |
50.9 |
87.9 |
71.5 |
|
Hyper |
70.0 |
72.6 |
51.4 |
85.5 |
71.9 |
|
Presence of coagulation necrosis sign |
66.2 |
76.5 |
53.8 |
84.6 |
73.5 |
|
Absence of calcification |
100 |
15.0 |
33.0 |
100 |
40.0 |
EBUS: endobronchial ultrasound; PPV: positive predictive value; NPV: negative predictive value.
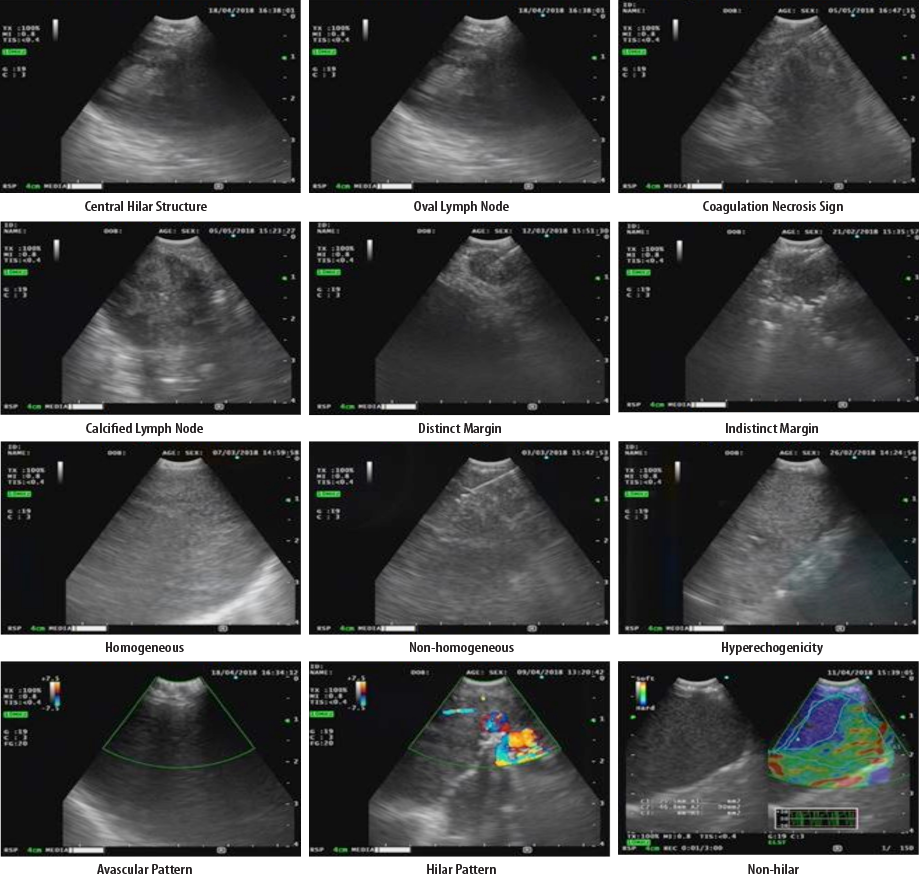
Figure 2: Morphological findings of endobronchial ultrasound characteristics.
Discussion
EBUS, which has been used widely in recent years, can be performed with high sensitivity and quality by expert physicians. This method is used to diagnose both benign and malignant diseases as well as to evaluate indistinguishable indeterminate lymphadenopathy.2 Although the efficiency of EBUS modalities in the investigation of mediastinal and hilar LNs has been proven, there is a need for further studies to find a consensus on all features of malignant or non-malignant LNs. Considering the epidemiological differences in the distribution of non-malignant cases in Iran, and the higher prevalence of TB and anthracosis, our study focused on some of the less-studied aspects of this issue.
Our study has revealed several important findings. It showed that size, shape, vascular pattern, echogenicity, margin, coagulation necrosis sign, calcification, and CHS are important criteria in distinguishing between malignant and non-malignant LNs. Of those, size > 1 cm, heterogeneous echogenicity, hyperechogenicity, the existence of necrosis signs, and the absence of CHS were predictive factors for the diagnosis of malignant LNs. All malignant LNs had at least one of these independent factors.
The mentioned findings are in agreement with previous studies in which the increase in the size of LNs was considered a predictor of malignancy.9,12,16–21 Heterogeneous echogenicity and the lack of CHS were other malignancy predicting factors that are independent of other factors and have been reported in other studies, previously.9,16,22
Our findings have several similarities and differences compared to an investigation of the sonographic features of LNs.16 In this study, four features (nodal size ≥ 10 mm, round shape, heterogeneous echogenicity, and the absence of CHS) were found to be predictive of malignancy predictive factors.16 The lack of CHS in malignant nodes occurs due to considerable increases in size that prevent the hilum from being seen.23 Although the round shape was seen significantly more often among the malignant group in our study, this factor was not an independent factor in predicting malignancy. This may occur because of the small sample size and the unequal distribution of patients in the two groups.
Another study evaluated 1061 LNs in 487 cancer cases from 2003 to 2007, retrospectively.9 Their study revealed that a round shape, margins of distinct, heterogeneous echogenicity, and the existence of CHS were independent factors for predicting metastasis. While, in our study, nodal size, hyperechogenicity, and the absence of CHS were found to be significant factors, and a round shape and distinct margins on EBUS were ruled out as an independent feature of malignancy. Well-defined margins on EBUS can be considered as predictors of benign disease.24 The differentiation between distinct and indistinct margin is made based on an expert’s judgment, and contradictory findings in different studies in this regard may be due to different opinions and/or human error.24
From 2008 to 2010, 100 patients with defined malignancy were studied with the size and shape of LNs, both round and oval, considered as the predictive factors of metastasis in the mediastinum.12 Echogenicity and border contour on EBUS were not correlated with malignancy. Therefore, in our study, a distinct margin and echogenicity, but not round and oval shape, were found to be predictors of malignancy.
In a study including 93 patients, EBUS-TBNA had a sensitivity of 85% and a NPV of 76% for the diagnosis of malignant LNs.25 In addition, in 2012, a similar study on 117 patients with extrathoracic malignancy reported a sensitivity of 86.4% and an NPV of 75%.26 In our study, the sensitivity and the NPV were calculated at 78.2% and 88.1%, respectively. Additionally, the diagnostic accuracy was 64.3%. The best sensitivity for predicting malignancy is size > 1 cm (sensitivity = 94.6%). The NPV of size was 90.2% while the specificity of this feature was poor (20.7%). Moreover, the second-highest sensitivity (81.1%) belonged to the absence of CHS with a specificity of 59.0% and an NPV of 88.3%. The absence of CHS also had the highest sensitivity in another study.10
Performing biopsy with suction does not affect diagnostic yield, which was consistent with the findings of a previous study in which the authors concluded that EBUS-TBNA, both with and without suction, are known as reliable techniques.27
We encountered several limitations in our study. For example, rapid, on-site evaluation was not present, and some samples were invalidated due to not having enough cell quantity to be diagnostic. In addition, the unavailability of high-quality needles in the country due to different sanctions imposed on Iran added further limitations to the study. Therefore, many samples were lost because we could not confirm the diagnosis. The uneven distribution of patients in the malignant and non-malignant groups was the other limitation of our study. As our institute is a referral center for tuberculosis and sarcoidosis, the uneven distribution may contribute to this issue. Despite all the mentioned limitations, the current report revealed promising results in using EBUS-TBNA for distinguishing malignancy and benignity of LNs.
Conclusion
Our study reveals that sonographic features achieved by EBUS-TBNA, as an easy, low-hazard, and precise technique, are factors features that can be reliably used to distinguish malignant from non-malignant LNs.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Sehgal IS, Agarwal R, Dhooria S, Prasad KT, Aggarwal AN. Role of EBUS TBNA in staging of lung cancer: a clinician’s perspective. J Cytol 2019 Jan-Mar;36(1):61-64.
- 2. Satterwhite LG, Berkowitz DM, Parks CS, Bechara RI. Central intranodal vessels to predict cytology during endobronchial ultrasound transbronchial needle aspiration. J Bronchology Interv Pulmonol 2011 Oct;18(4):322-328.
- 3. Muthu V, Sehgal IS, Dhooria S, Prasad KT, Gupta N, Aggarwal AN, et al. Endobronchial ultrasound-guided transbronchial needle aspiration: techniques and challenges. J Cytol 2019 Jan-Mar;36(1):65-70.
- 4. Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA; American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007 Sep;132(3)(Suppl):202S-220S.
- 5. Chandra S, Nehra M, Agarwal D, Mohan A. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle biopsy in mediastinal lymphadenopathy: a systematic review and meta-analysis. Respir Care 2012 Mar;57(3):384-391.
- 6. Wong M, Yasufuku K, Nakajima T, Herth FJ, Sekine Y, Shibuya K, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J 2007 Jun;29(6):1182-1186.
- 7. Tremblay A, Stather DR, MacEachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009 Aug;136(2):340-346.
- 8. Nakajima T, Yasufuku K, Kurosu K, Takiguchi Y, Fujiwara T, Chiyo M, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis–comparisons with other bronchoscopic diagnostic modalities. Respir Med 2009 Dec;103(12):1796-1800.
- 9. Fujiwara T, Yasufuku K, Nakajima T, Chiyo M, Yoshida S, Suzuki M, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010 Sep;138(3):641-647.
- 10. Wang L, Wu W, Hu Y, Teng J, Zhong R, Han B, et al. Sonographic features of endobronchial ultrasonography predict intrathoracic lymph node metastasis in lung cancer patients. Ann Thorac Surg 2015 Oct;100(4):1203-1209.
- 11. Bhowmik A, Herth FJ. Bronchoscopy and other invasive procedures for diagnosis. Tuberculosis (Edinb) 2018;82:137.
- 12. Wang Memoli JS, El-Bayoumi E, Pastis NJ, Tanner NT, Gomez M, Huggins JT, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011 Dec;140(6):1550-1556.
- 13. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. Journal of thoracic oncology. 2009;4(5):568-577.
- 14. Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997 Jun;111(6):1718-1723.
- 15. Yasufuku K, Nakajima T, Motoori K, Sekine Y, Shibuya K, Hiroshima K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006 Sep;130(3):710-718.
- 16. Jhun BW, Um S-W, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Clinical value of endobronchial ultrasound findings for predicting nodal metastasis in patients with suspected lymphadenopathy: a prospective study. J Korean Med Sci 2014 Dec;29(12):1632-1638.
- 17. Libshitz HI, McKenna RJ Jr. Mediastinal lymph node size in lung cancer. AJR Am J Roentgenol 1984 Oct;143(4):715-718.
- 18. Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol 1985 Feb;144(2):261-265.
- 19. Genereux GP, Howie JL. Normal mediastinal lymph node size and number: CT and anatomic study. AJR Am J Roentgenol 1984 Jun;142(6):1095-1100.
- 20. Lee N. [Endoscopic ultrasonography for preoperative diagnosis of the hilar and mediastinal lymph node metastases in lung cancer] (Zasshi). (Journal). Nihon Kyobu Geka Gakkai Zasshi 1992 Jun;40(6):937-945.
- 21. Lee N, Inoue K, Yamamoto R, Kinoshita H. Patterns of internal echoes in lymph nodes in the diagnosis of lung cancer metastasis. World J Surg 1992 Sep-Oct;16(5):986-993, discussion 993-994.
- 22. Schmid-Bindert G, Jiang H, Kähler G, Saur J, Henzler T, Wang H, et al. Predicting malignancy in mediastinal lymph nodes by endobronchial ultrasound: a new ultrasound scoring system. Respirology 2012 Nov;17(8):1190-1198.
- 23. Hayat M. Cancer imaging: instrumentation and applications: Academic Press; 2007.
- 24. Ayub II, Mohan A, Madan K, Hadda V, Jain D, Khilnani GC, et al. Identification of specific EBUS sonographic characteristics for predicting benign mediastinal lymph nodes. The Clinical Respiratory Journal 2016;12(2):681-690.
- 25. Tournoy KG, Govaerts E, Malfait T, Dooms C. Endobronchial ultrasound-guided transbronchial needle biopsy for M1 staging of extrathoracic malignancies. Ann Oncol 2011 Jan;22(1):127-131.
- 26. Sanz-Santos J, Cirauqui B, Sanchez E, Andreo F, Serra P, Monso E, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies. Clin Exp Metastasis 2013 Apr;30(4):521-528.
- 27. Harris K, Maroun R, Attwood K, Chalhoub M. Comparison of cytologic accuracy of endobronchial ultrasound transbronchial needle aspiration using needle suction versus no suction. Endosc Ultrasound 2015 Apr-Jun;4(2):115-119.