Rheumatoid arthritis (RA) is a chronic systemic musculoskeletal disease in which various joints in the body are inflamed, leading to swelling, pain, stiffness, and a possible loss of function.1,2 RA is caused by an imbalance in the number or function of T helper 17 (Th17) cells and regulatory T (Treg) cells.3,4 Increased oxidative stress also participates in the pathogenesis and severity of the disease.5,6
Oxidative stress in RA can be caused by deteriorating oxygen supply to the joint tissues thereby supporting increased reactive oxygen species (ROS), causing oxidative damage that further promotes inflammation. Hence, oxidative stress and inflammation are inseparably connected.7,8 Hypoxia-inducible factor-1 alpha (HIF-1α) is a key transcriptional regulator that enables cellular metabolic adaptation to low levels of oxygen.9 As a transcription factor, it influences and regulates the expression of dozens of genes involved in maintaining homeostasis such as changes in oxygen concentration (oxygen-dependent) and independent signals.10,11 Superoxide dismutase (SOD) antioxidant is a protein that functions as important defense mechanisms for oxidative stress and acts as a first-line component of the defense system against free radicals or reactive species.12
Interleukin 17a (IL-17a) is an inflammatory biomarker for RA, which has a strong association with C-reactive protein (CRP).13 CRP is a member of the pentraxins protein family, which is composed of five 23-kDa subunits and its levels can increase by 1000-fold or more with infection, inflammation, and tissue injury. CRP levels correlate with morning stiffness, pain, fatigue, grip strength, articular index, and disability.14
Rheumatoid factor (RF) is the most common laboratory serologic marker for the diagnosis of RA.15 Although RF can be detected in patients with other connective tissue diseases, RF isotypes are helpful in the management of RA patients from the time of diagnosis until deciding on the choice of therapeutic strategy. RF testing in RA patients has a sensitivity of 60% to 90% and a specificity of 85%.16
RA causes high-intensity pain and severe suffering because it is often not diagnosed or treated quickly enough.17 Joint destruction occurs in the first six months after illness, and permanent defects occur two to three years later if left untreated.18 Extra-articular manifestations of RA occur in 17.8–40.9% of RA patients and 1.5–21.5% are usually associated with increased morbidity and mortality such as cardiopulmonary and kidney due to developing the disease itself or the effects of the drugs given.19,20 Years of aggressive treatment are needed to control symptoms, manage pain, and stop the development of RA. Standard therapy in the form of drugs and even surgery has not provided optimal results.21
Hyperbaric oxygen therapy (HBOT) is based on administering pure oxygen to the patient while undergoing increased ambient pressure.22 HBOT can be a new breakthrough for creating appropriate treatment strategies and reducing the adverse effects of the drugs used, so HBOT research on RA needs to be developed again. The Naval Health Institute of the Indonesian Navy has used HBOT to treat diving cases of decompression sickness, arterial gas embolism, and gas poisoning, and even as an adjunct therapy in some cases of clinical diseases such as wound healing, diabetes mellitus with gangrene gas, and osteomyelitis. Several studies have shown that HBOT has a specific mechanism that can reduce inflammation, but the mechanism of reducing arthritis in RA is still unclear.
Methods
We conducted a randomized, control study between January and April 2018. We used BALB/c mice, male, weighing 20–30 grams, aged 10–14 weeks, healthy during the adaptation phase with the characteristics of clear-eyed, shiny fur, agile movements, and good feces.
The nature of this experiment was invasive and fatal to the experimental animals. Random sampling was used to select 16 mice with collagen-induced antigen and arthritis (ACIA) from a total of 20. The selected mice were divided into two groups with each group consisting of eight mice. The first group was the control group (non-HBOT) and the second group was the treatment group (HBOT).
We used ACIA as an animal model of RA because ACIA is an animal model that is very similar to RA in humans. Mice were injected with 100 μg of methylated bovine serum albumin (mBSA) in 50 μL phosphate buffer saline (PBS), which was emulsified with 50 μL complete Freund adjuvant subcutaneously and 200 ng of Bordetella pertussis toxin (PTx) intraperitoneally.
Seven days later, mice were injected with 50 μg mBSA and 100 μg collagen type II (CII) in 50 μL PBS were emulsified with 50 μL of incomplete Freund adjuvant (IFA) and 200 ng PTx subcutaneously. On day 14 of the study, mice were injected with 50 μg mBSA and 100 μg CII in 50 μL PBS emulsified with 50 μL IFA subcutaneously and 200 ng PTx intraperitoneally. On day 28 of the study, mice were induced with 50 μg mBSA dissolved in 20 µL PBS into the left cavity of the knee joint intra-articular (ipsilateral) and the right knee cavity (contralateral) was injected with 20 μL PBS intraarticularly. Twenty-one days later (day 49 of the study), we got research animals with the characteristics of RA disease (ACIA animal models).
We used Treatment Table 9 (USN TT9), the hyperbaric oxygen table dosing protocol developed by the United States Navy (USN). The treatment group was given normal air exposure for 10 minutes at 2.4 atmospheres absolute (ATA) pressure. Then given oxygen exposure of 100% 3–4 L/min for 90 minutes divided by 3 × 30 minutes intervals 2 × 5 minutes breathing with normal air at 2.4 ATA pressure. After that, the pressure was reduced to 1 ATA while breathing normal air without using oxygen for 10 minutes. The treatment group received HBOT for 10 consecutive days.
All mice were anesthetized with ketamine (300 mg/kg body weight + xylazine 40 mg/kg body weight) intraperitoneally after 30 minutes of exposure to HBO. Blood and joint tissue samples were taken after 10 minutes to ensure no pain response. Blood was taken using a syringe on the heart ventricle for enzyme-linked immune-sorbent assay (ELISA) examination, and synovial tissue was taken by using a scalpel for immunohistochemical examination. The mice were killed by neck dislocation.
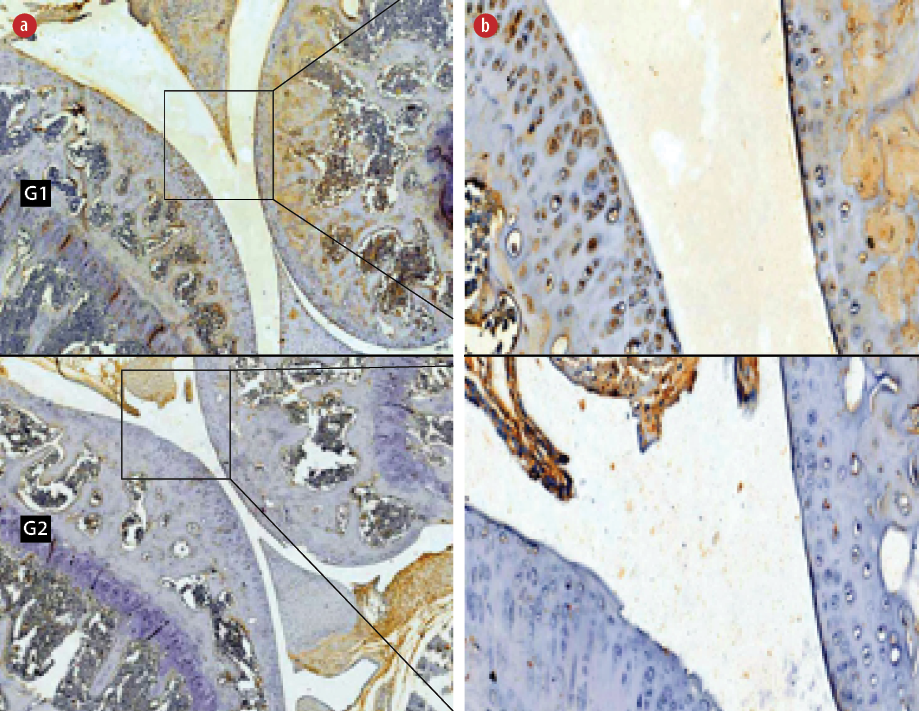
Figure 1: Immunohistochemical staining of hypoxia-inducible factor-1α (HIF-1α) in the (G1) non-hyperbaric oxygen therapy (HBOT) and (G2) HBOT groups. Living cells that expressed the HIF-1α protein (positive cells) were stained brown and blue cells (hematoxylin stain) showed no expression of the HIF-1α protein. Magnification (a) = 50 ×, (b) = 400 ×.
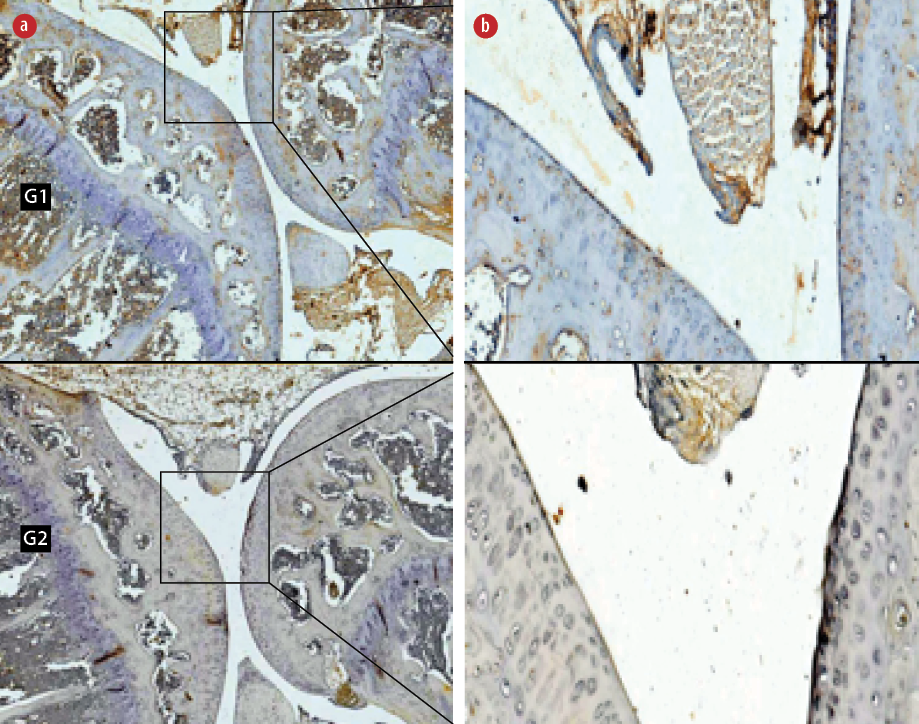
Figure 2: Immunohistochemical staining of anti-cluster differentiation 196 (CD196) in the (G1) non-hyperbaric oxygen therapy (HBOT) and (G2) HBOT groups. Living cells that expressed the CD196 protein (positive cells) were stained brown and blue (hematoxylin stain) cells showed no expression of the CD196 protein. Magnification (a) = 50 ×, (b) = 400 ×.
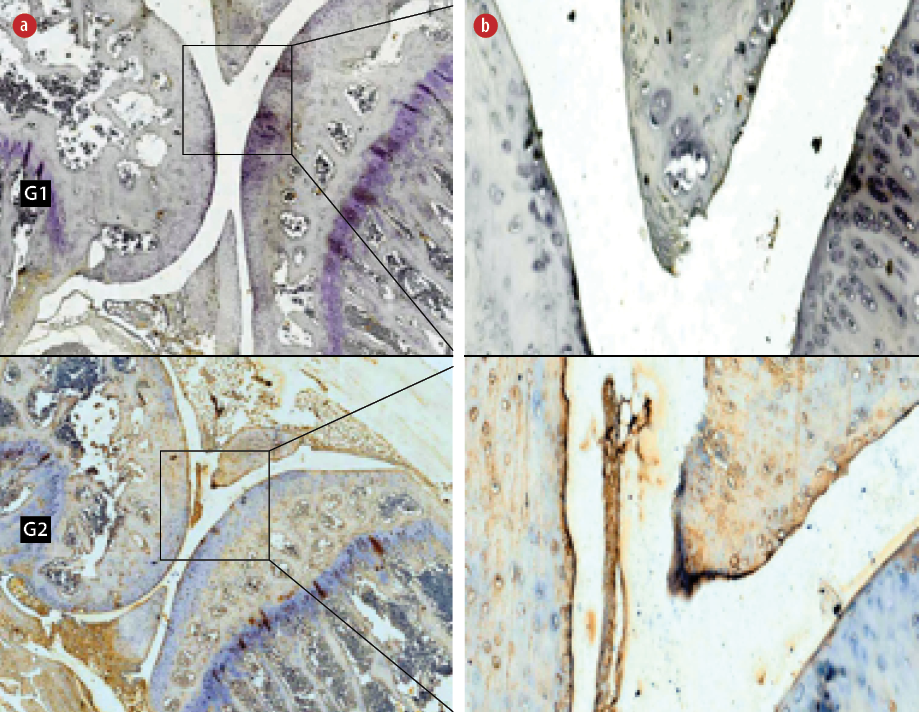
Figure 3: Immunohistochemical staining of anti-interleukine 2 receptor β-chain cells (IL-2Rβ) in the (G1) non-hyperbaric oxygen therapy (HBOT) and (G2) HBOT groups. Living cells that expressed the IL-2Rβ protein (positive cells) were stained brown and blue cells (hematoxylin stain) showed no expression of the
IL-2Rβ protein. Magnification (a) = 50 ×, (b) = 400 ×.
The expression of HIF-1α was determined using the anti-HIF-1α mouse monoclonal immunoglobulin G1 (IgG1) antibody, the anti-cluster differentiation 196 (CD196) mouse monoclonal IgG1 antibody for the expression of Th17, and anti-interleukine 2 receptor β-chain cells (IL-2Rβ) mouse monoclonal IgG2b antibody (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for expression of Treg. We used the avidin-biotin complex for staining. Immunoexpression was calculated from the average number of brown cells in five large visual fields at 400 × magnification on a light microscope.
The level of serum RF was measured using IgG RF Mouse ELISA kit (LifeSpan BioSciences, Inc., USA). The level of serum CRP was measured using mouse CRP antibody kit (LifeSpan BioSciences, Inc., USA), and level of IL-17a in plasma was measured using mouse IL-17a antibody (Bioassay Technology Laboratory system, Korain Biotech Co., Ltd, Shanghai, China) with an enzyme-linked immunosorbent assays (ELISA) reader (Zenix-320 microplate reader). The examination of plasma SOD levels was measured using a mouse SOD ELISA kit (LifeSpan BioSciences, Inc., USA) with an ELISA reader (Spectrophotometer Zhimadzu) analyzers. The concentrations of RF (IU/mL), CRP (μg/mL), IL-17a (ng/mL), and SOD (μL/mL) captured were determined by measuring absorbance at 450 nm using a spectrophotometer.
The degree of arthritis was assessed by clinical scoring of paw swelling and the diameter of the paw was measured by digital calliper (Mitutoyo, Japan). The clinical scoring of the paw was graded as follows: 0 = normal and no swelling; 1 = erythema and mild edema; 2 = erythema and moderate edema; 3 = erythema and severe edema; 4 = maximal swelling and deformation leading to incapacitated limb. The calliper was placed across the paw at widest point with a level of accuracy up to 0.05 mm (the number of strips on the slider scale was 20 so that 1 mm: 20 = 0.05 mm). The values were expressed as the mean of the two paw diameters of mice.
All data were analyzed using SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). All values were expressed as mean±standard deviations (SD). A value of p < 0.050 was considered statistically significant.
Results
The normality test, using the Shapiro-Wilk test showed the expression of HIF-1α, CD196, IL-2Rβ, SOD levels, IL-17a, CRP, RF, clinical scoring of paw swelling, and diameter of paw swelling had normal distribution (p > 0.050). The results of Lavene test showed expression of HIF-1α, SOD levels, IL-17a, RF, and the clinical scoring of paw swelling and the diameter of paw swelling had homogeneous variance (p > 0.050), but the expression of CD196, IL-2Rβ, and the level of CRP did not have homogeneous variance (p < 0.050).
The independent t-test showed there was a significant decrease in the expression of HIF-1α in HBO group (8.7±2.2, p < 0.001) compared to non-HBO group (69.9±3.1, p < 0.050) [Figure 1].
The Mann-Whitney test determined that the expression of CD196 also decreased significantly in the HBO group (4.8±1.4, p < 0.001) compared to non-HBO group (40.0±4.7, p < 0.050) [Figure 2].
The Mann-Whitney test showed the expression of IL-2Rβ increased significantly in the HBO group (5.0±1.3, p < 0.001) compared to the non-HBO group (48.2±17.9, p < 0.050) [Figure 3].
The differences in the results of oxidative stress, systemic inflammation, and degree of arthritis in experimental groups are shown in Table 1. In the independent t-test showed there was no significant increase (p = 0.093) in SOD levels, there was a significant decrease (p < 0.001) in the level of IL-17a, RF, and clinical scoring of paw swelling in the treatment group compared to control group. The diameter of paw swelling also decreased significantly in the treatment group compared to the control group [Table 1]. There was no significant decrease in CRP levels between the two groups.
Table 1: Differences of the results of systemic inflammation, oxidative stress, and degree of arthritis in the control and treatment groups.
SOD: superoxide dismutase; IL-17: interleukin 17; CRP: C-reactive protein; RF: rheumatoid factor.
Data shown as mean±SD; p < 0.050 was considered statistically significant.
G1: non-HBOT group; G2: HBOT group.
Research on RA continues to develop. Research on the use of antioxidants or antioxidant-containing foods combined with drugs, such as methotrexate, and the addition of natural polyphenol antioxidants, including silibinin can function to increase the effect of drugs and reduce oxidative stress.23,24 Our study limited the use of HBOT alone to determine the effect and basic mechanism of oxygen use on the improvement of hypoxic cells and to see the extent of the role of HBOT in polarization or changing of phenotype from Th17 cells to Treg cells. In the future, research on the use of HBOT in combination with drugs, biological agents, or other antioxidants needs to be considered and investigated further.
Several HBOT studies on RA have been carried out. Clinical improvement due to decreased serum immune complexes and T lymphocyte function was found in patients with RA after 21 sessions of HBOT under 1.7 ATA for 40 minutes.25 HBOT 70% at 1.5 ATA for three hours daily for two weeks reduced neuropathic pain in eight Sprague-Dawley male mice RA models.26 In contrast to previous studies, this study examined the strategy of autoimmune anti-rheumatic therapy involving polarization of Th17 cells (CD196) into Treg (IL-2Rβ) through changes in expression of HIF-1α after HBOT. This study used higher oxygen levels and pressure than previous studies but was still based on safe doses. It has been suggested that if the pressure used to deliver hyperbaric oxygen did not exceed 3 ATA (equivalent to 20 meters in seawater) and the duration of treatment for elective therapy did not exceed two hours then this HBOT was still considered safe.27
This study used HBOT with dosage according to USN TT9 breathing 100% oxygen for 3 × 30 minutes intervals 2 × 5 minutes breathing normal air at 2.4 ATA for 10 consecutive days. Higher doses were expected to provide more optimal results. Some theories stated that HBOT was considered a safe treatment modality but carried a risk because of hyperoxia and increased pressure. HBOT acted as a chemical agent that could affect the oxidants and antioxidants system. The main mechanism for HBOT was based on the generation of intracellular ROS. Previously, it has been stated that respiration with high oxygen concentrations and pressure greater than 1 ATA would increase ROS production.28,29 We thought that HBOT could lead to increased ROS and oxidative stress if given at excessive doses; therefore, this study was limited to 10 consecutive days.
Studies of the effects of HBOT on RA on oxidative stress has also been carried out previously using oxygen levels and lower pressures compared to this study.30,31 HBOT increased the SOD activity, decreased the value of lipoperoxide, and improved the erythrocyte sedimentation rate and the Lansbury articular index in patients with RA.30 HBO oxygen 36% at 1.25 ATA for three weeks reduced derivative reactive oxygen metabolites and CRP in collagen-induced arthritis type II mice.31
The results of the study on oxidative stress levels could be seen in SOD levels. The level of SOD increased but not significantly in the HBOT group compared to non-HBOT group. We hypothesized that HBOT increased ROS production but, if the level was not excessive, it could be useful because ROS could also act as a cellular messenger in many signal transduction pathways and induce other cytoprotective genes.32,33 This seemingly controversial effect was strongly influenced by therapeutic doses, and the length and interval of exposure. ROS was related to the amount of oxygen present, but HBOT paradoxically induced the activity of antioxidant enzymes such as SOD. The nuclear factor erythroid 2-related factor 2 (Nrf2)/Keap1 pathway was the main regulator of redox homeostasis. Nrf2 had a major contribution to the regulation of defense systems in various antioxidants as a cytoprotective response to endogenous and exogenous pressures caused by ROS.34 SOD functioned to suppress or prevent the formation of ROS in cells by rapidly neutralizing any molecule with the potential to develop into free radicals or any free radicals with the ability to induce other radical production.35 This fact was consistent with the concept of mitochondrial hormesis, which states the production of ROS could induce a positive response that was increased resistance to stress, and actually cause oxidative stress to decrease.36
Understanding HIF expression in RA joints allows us to better understand the level at which they are activated based on the severity of the disease, and how it affects certain cell types that contribute to perpetuating this disease. RA triggers the accumulation of HIF-1α and HIF-2α chains, so that the target of RA therapy was HIF-1α and HIF-2α.37 We chose the HIF-1α variable instead of HIF-2α in this study because a previous study stated that HIF-1α was widely expressed and was believed to play an important role in the hypoxic response compared to HIF-2α.38 Under normoxia or hyperoxia conditions, HIF-1α was more easily degraded than HIF-2α, which was more stable.39,40 After HBOT, HIF-1α was hydroxylated by prolyl hydroxylase domain proteins, recognized by the ubiquitin E3 ligase, and directed to the proteasome for degradation.41,42
The results of this study showed significant decreases in HIF-1α and Th17 (CD196) expression, and a significant increase in the expression of Treg (IL-2Rβ) in the treatment group compared to the control group. The mechanism of HBOT hyperoxia or normoxia after exposure to HBOT caused HIF-1α activity to decrease, which caused polarization or differentiation of phenotypes from Th17 (CD196) to Treg (IL-2Rβ). Decreasing the expression of HIF-1α activated FOXP3 gene expression and finally degraded RORγt in Th17/Treg progenitor when committing to polarization from Th17 to Treg so that the number of Th17 (CD196) decreased.
IL-17a levels decreased significantly and CRP levels decreased but not significantly in the treatment group compared to the control group. This is in accordance with previous studies that found a relationship between serum IL-17a levels and CRP. IL-17a is inducer CRP from mouse smooth muscle cells and hepatocytes.43,44 CRP levels decreased, but not significantly, because many factors also influence CRP levels. ROS is also thought to play a role in increasing CRP levels.45 We suspect that ROS as a result of exposure to HBOT could also play a role in CRP metabolism. Increased blood levels of IL-17a from mice with RA were of limited use as biomarkers to show disease activity.46
RFs are a family of autoantibodies directed to the Fc portion of IgG. They are locally produced in RA by B cells present in lymphoid follicles and germinal center-like structures that develop in inflamed synovium.47 They are heterogeneous and usually composed of IgM. Because of this, most assays detect only IgM. RFs are used as a marker in individuals with suspected RA or other autoimmune conditions. Detection of IgM RFs is also helpful as a prognostic index, and some studies have shown that immunosuppressive treatment can decrease serum RF levels. However, the clinical usefulness of RFs in monitoring disease activity and treatment response is limited. In this study, the level of IgM RF decreased significantly in the treatment group compared to the control group. Decreasing the number of Th17 after exposure to HBOT caused a significant decrease in IL-17a production in the treatment group compared to the control group. This resulted in a direct decrease in autoreactive B cell proliferation and a decrease in differentiation and plasma cell activity,16 which resulted in a decrease in the production of RF.
Hypoxia had been shown to induce an inflammatory response, in this case, ACIA. The reduction of arthritis clinically after exposure to HBOT could be seen from the results of clinical scoring and diameter of paw swelling, which both decreased significantly in the treatment group compared to the control group. The decrease in the amount and function of Th17 caused a decrease in the production of pro-inflammatory cytokines IL-17a, IL-17f, IL-21, IL-22, interferon γ, and granulocyte-macrophage-colony-stimulating factor so the arthritis decreased.48
We recommend the use of HBOT as a supporting therapy in RA. Our study provides new insights into therapeutic interventions in human autoimmune diseases.
The authors declared no conflict of interests. The Ethics Committee of Naval Health Institute, Indonesian Navy stated that this study was feasibly approved (Animal Ethical Clearance Certificate No.009/AECC/NHI/IX/2017). This study received support from the Department of Physiology, Faculty of Medicine, Hang Tuah University, Surabaya, Indonesia; Department of Hyperbaric, Drs. Med. R. Rijadi S., Phys. Naval Health Institute, Indonesian Navy, Surabaya, Indonesia; and Department of Biochemistry, Unit of the Experimental Animal, Faculty of Medicine, Airlangga University, Surabaya, Indonesia. This research was funded by the Faculty of Medicine, Hang Tuah University, Surabaya, Indonesia.