Eosinophilic fasciitis (EF) is a rare systemic inflammatory disease with unknown etiology characterized by symmetrical swelling and induration of the skin, sparing the distal parts of the arms and/or legs, evolving into a scleroderma-like appearance, accompanied by peripheral blood eosinophilia. The diagnosis is further confirmed by a full-thickness skin biopsy and/or magnetic resonance imaging (MRI). Corticosteroid treatment remains the standard therapy, either alone or with an immuno-suppressive drug.1
Case report
A 41-year-old woman with asthma (well-controlled on inhalers), hypothyroidism (controlled on thyroxin), and gastroesophageal reflux disease presented to the rheumatology clinic at the Royal Hospital with a one-month history of bilateral swelling of the forearms with skin tightness and fingers contraction. She had no constitutional symptoms or history of Raynaud’s phenomena, weight loss, or change in her bowel habits. Physical examination revealed edema and hardening of the subcutaneous tissue of the forearm. The skin of both forearms showed a linear depression along the course of the superficial veins consistent with groove sign. She was unable to flex or extend her fingers and to make a fist or hold objects well. The skin over her fingers and palms was normal. Her face was unaffected. There were no clinical features suggestive of malignancy or infection. Laboratory tests revealed raised eosinophil count of 1.8 × 109/L (normal range 0–0.5 × 109 g/L). Her level of C-reactive protein was mildly raised; creatine kinase and erythrocyte sedimentation rate (ESR) were normal. Rheumatoid factor, anti-nuclear antibody, and extractable nuclear antigen and lactate dehydrogenase were negative. Full-thickness biopsy of the skin and muscles of the forearms showed inflammatory process involving the interstitial tissue in and around the skeletal muscle along with occasional muscle necrosis and some regenerative fibers with an increased number of eosinophils in the fascia fibroconnective tissue [Figure 1 a-f], which confirmed EF. Contrast MRI revealed extensive bilateral enhancing thickened fascia between the muscles of the forearm [Figure 1 g-i]. She was started on oral prednisolone 0.75 mg/kg for four weeks, which was subsequently slowly tapered. She made a remarkable response with reduced limb swelling and normal mobility. Unfortunately, the disease relapsed on tapering and high dose prednisolone was restarted along with adding oral methotrexate 20 mg per week. Her disease responded well to treatment; however, she was lost to follow-up and stopped the medication resulting in recurrence of her disease.
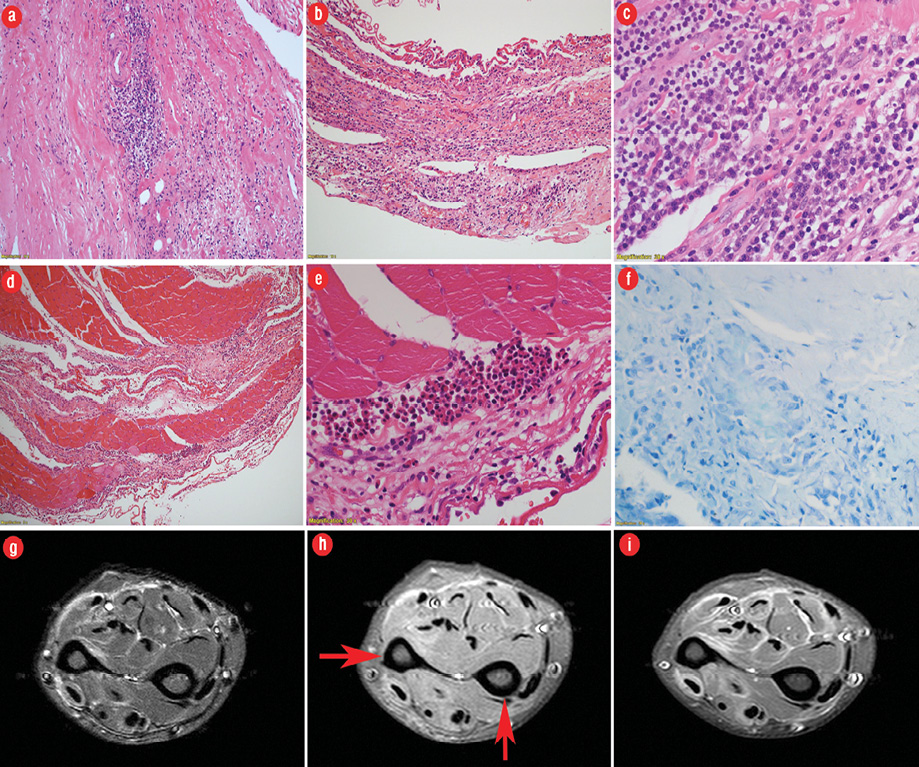
Figure 1: (a) Hematoxylin and eosin (H&E) staining of fascia showing perivascular and interstitial chronic inflammation and fibrosis (magnification = 200 ×). (b) H&E staining showing intense laminar chronic inflammatory reaction (magnification = 200 ×). (c) H&E staining showing dense chronic inflammation including numerous plasma cells, lymphocytes, and occasional macrophages (magnification = 600 ×). (d) H&E staining showing secondary involvement of skeletal muscle which shows few pale degenerate myofibres and extensive perimysial infiltration by chronic inflammatory cells (magnification = 100 ×). (e) H&E staining of skeletal muscle showing perimysial eosinophilic microabscess formation (magnification = 200 ×). (f) Ziehl–Neelsen staining was negative for acid-fast bacilli in granuloma (magnification = 600 ×). (g) Axial fat-suppressed, T2-weighted fast spin-echo MRI reveals markedly increased signal intensity within superficial and deep fascial layers and mildly increased T2 signal intensity within superficial muscle fibers adjacent to fascia. (h) Axial fat-suppressed T1-weighted spin-echo MRI shows prominent superficial and deep fascial thickening (arrows) with slightly increased signal intensity relative to muscle. (i) Axial enhanced, fat-suppressed, T1-weighted spin-echo MRI revealed intense fascial enhancement corresponding to locations of T2 signal abnormality.
Discussion
The etiology of EF is unknown. It has been reported after localized trauma, intense exercise, autoimmune disease (such as thyroid disease), and infection with Borrelia burgdorferi.2–4 EF may be associated with hematological disorders like aplastic anemia.5–9 It has also been reported in association with solid organ tumor, and post-allogeneic bone marrow transplant.10 Bronchial and allergic asthma has been reported in the literature with EF.11
EF affects the limbs and spares the face and hands, it usually begins with painful swelling, and tightening of the limbs, which within weeks to months progresses to fibrosis leading to flexion contractures and limited mobility.1 Groove sign (a depression along the course of the superficial veins seen best when elevating the affected limb) is typically found in EF, and its presence distinguishes EF from scleroderma in the absence of Raynaud’s phenomenon.12
Peripheral blood eosinophilia is seen in the majority of patients with EF, though not necessary for making the diagnosis. Around half of patients have elevated ESR and hypergammaglobulinemia. Serum anti-nuclear antibodies are not present.
A full-thickness biopsy (including skin, fascia, and muscle) is the gold-standard test for diagnosing EF.13,14 Involvement of the deep dermis and fascia is typical of the disease and is useful in excluding disease mimickers such as scleroderma and scleroderma-like syndromes. In the early stages of the disease edema of the deep subcutaneous tissue and fascia along with lymphocytic, plasma cell, histiocytic and eosinophilic infiltrates is usually seen. Later, collagen thickening and sclerosis of the deep dermis and fascia occurs with disappearance of the inflammatory cell infiltrates. Thickening and inflammation can also be seen in the adjacent muscle.
According to a recent study, EF may be histopathologically distinguished from localized scleroderma (morphea) based on helper T-cell (Th) subtype polarization where Th1/Th2 and the presence of Th17+ cells were significantly higher in EF compared to morphea, while the CD4/CD8+ T-cell ratio was significantly greater in morphea.15 If the biopsy is not possible to obtain or inconclusive, then MRI of the affected area may be used to confirm fascial inflammation.16
Due to the rarity of the disease, there is limited evidence in the therapeutic efficacy in treating this disease. The treatment is mainly dependent on expert opinion, case reports and case series. Steroids at a high dose (1–1.5 mg/kg) remain the first-line treatment.17 The issue with steroid is its complications on the short- and long-term period of treatments. Hence, there is a need to introduce other immunosuppressive medications such as steroid-sparing agents. Methotrexate was highly used in some case series and as intuitive because of its known safety profile in rheumatic disease.18
Other treatment possibilities that have been used in the treatment of EF are mycophenolate mofetil, hydroxychloroquine, sulfasalazine, and azathioprine.19 Biological agents like infliximab and rituximab have also been used in few cases refractory to the usual treatment with good response.20,21 Surgical intervention is sometimes needed in cases where the response to steroid is inadequate or treatment delays for release of joints contractors.22
In our case, there was a clear response to the high dose steroid, which was working well as induction for the therapy and the methotrexate was used to maintain the disease remission. However, the disease relapsed upon treatment discontinuation by the patient. This emphasizes the need for long-term follow-up of these patients by rheumatologist and treatment tapering and cessation should be done carefully.
Conclusion
EF is a rare systemic inflammatory disease with unknown etiology. Clinical examination is necessary for the diagnosis of EF in the presence of peripheral eosinophilia. It is further confirmed by histopathological examination. Systemic glucocorticoids are the first-line treatment. In relapsing or resistant cases, immunosuppressive drugs can be used.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Lamback EB, Resende FS, Lenzi TC. Eosinophilic fasciitis. An Bras Dermatol 2016 Sep-Oct;91(5)(suppl 1):57-59.
- 2. Hashimoto Y, Takahashi H, Matsuo S, Hirai K, Takemori N, Nakao M, et al. Polymerase chain reaction of Borrelia burgdorferi flagellin gene in Shulman syndrome. Dermatology 1996;192(2):136-139.
- 3. Mosconi S, Streit M, Brönimann M, Braathen L. Eosinophilic fasciitis (Shulman syndrome). Dermatology 2002;205(2):204-206.
- 4. Granter SR, Barnhill RL, Duray PH. Borrelial fasciitis: diffuse fasciitis and peripheral eosinophilia associated with Borrelia infection. Am J Dermatopathol 1996 Oct;18(5):465-473.
- 5. Haddad H, Sundaram S, Magro C, Gergis U. Eosinophilic fasciitis as a paraneoplastic syndrome, a case report and review of the literature. Hematol Oncol Stem Cell Ther 2014 Jun;7(2):90-92.
- 6. Doyle JA, Connolly SM, Hoagland HC. Hematologic disease in scleroderma syndromes. Acta Derm Venereol 1985;65(6):521-525.
- 7. Hoffman R, Dainiak N, Sibrack L, Pober JS, Waldron JA Jr. Antibody-mediated aplastic anemia and diffuse fasciitis. N Engl J Med 1979 Mar;300(13):718-721.
- 8. Hiraiwa T, Mori T, Ohashi T, Hanami Y, Yamamoto T. Eosinophilic fasciitis with severe joint contracture in a patient with bladder cancer and B-cell lymphoma. J Dermatol 2016 Jan;43(1):68-69.
- 9. Khanna D, Verity A, Grossman JM. Eosinophilic fasciitis with multiple myeloma: a new haematological association. Ann Rheum Dis 2002 Dec;61(12):1111-1112.
- 10. Chu GY, Lin HL, Chen GS, Wu CY. Eosinophilic fasciitis following allogeneic bone marrow transplantation in a patient with acute myeloid leukaemia. Acta Derm Venereol 2014 Mar;94(2):221-222.
- 11. Yamanishi Y, Ishioka S, Yamakido M. Complete remission of relapsing eosinophilic fasciitis associated with bronchial asthma following regular steroid inhalation. Rheumatology (Oxford) 2000 Mar;39(3):339-340.
- 12. Fruchter R, Mazori DR, Femia AN. Groove sign of eosinophilic fasciitis. J Clin Rheumatol 2017 Apr;23(3):169.
- 13. Das J, Chinoy H, Dick J, Matthews R, Roberts M. A Literature review of eosinophilic fasciitis with an illustrative case. Curr Rheumatol Rev 2017;13(2):113-120.
- 14. Mazori DR, Femia AN, Vleugels RA. Eosinophilic fasciitis: an updated review on diagnosis and treatment. Curr Rheumatol Rep 2017 Nov;19(12):74.
- 15. Moy AP, Maryamchik E, Nikolskaia OV, Nazarian RM. Th1- and Th17-polarized immune infiltrates in eosinophilic fasciitis-A potential marker for histopathologic distinction from morphea. J Cutan Pathol 2017 Jun;44(6):548-552.
- 16. Desvignes-Engelbert A, Saulière N, Loeuille D, Blum A, Chary-Valckenaere I. From diagnosis to remission: place of MRI in eosinophilic fasciitis. Clin Rheumatol 2010 Dec;29(12):1461-1464.
- 17. Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum 1988 May;17(4):221-231.
- 18. Lebeaux D, Francès C, Barete S, Wechsler B, Dubourg O, Renoux J, et al. Eosinophilic fasciitis (Shulman disease): new insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford) 2012 Mar;51(3):557-561.
- 19. Jones AC, Doherty M. Eosinophilic fasciitis with late onset arthritis responsive to sulfasalazine. J Rheumatol 1993 Apr;20(4):750-751.
- 20. Khanna D, Agrawal H, Clements PJ. Infliximab may be effective in the treatment of steroid-resistant eosinophilic fasciitis: report of three cases. Rheumatology (Oxford) 2010 Jun;49(6):1184-1188.
- 21. Scheinberg M, Hamerschlak N, Kutner JM, Ribeiro AA, Ferreira E, Goldenberg J, et al. Rituximab in refractory autoimmune diseases: Brazilian experience with 29 patients (2002-2004). Clin Exp Rheumatol 2006 Jan-Feb;24(1):65-69.
- 22. Suzuki G, Itoh Y, Horiuchi Y. Surgical management of eosinophilic fasciitis of the upper extremity. J Hand Surg Br 1997 Jun;22(3):405-407.