|
Introduction
A 65-year-old diabetic male on insulin injections for the past 15 years, hypertensive on amlodepine, dyslipidemic on statins and a nonsmoker was presented to the Emergency department with acute infero-posterior wall ST-elevation myocardial infarction along with complete heart block with heart rate of 40 beats per minute and blood pressure of 100/70 mmHg. A prophylactic transvenous temporary pacing wire was inserted from the right femoral vein after placing a femoral sheath. While attempting femoral venous cannulation, the right femoral artery was inadvertently punctured and a sheath was temporarily introduced and removed. Following the stabilization with a pacing wire, the patient was thrombolyzed with intravenous reteplase (10 units + 10 units, 30 minutes apart), followed by unfractionated heparin infusion (5000 units bolus + 1000 units/hour infusion). The patient responded well with a return of sinus rhythm at 70 beats per minute. The pacing wire along with the sheath was removed after 48 hours. His urea and creatinine were elevated at 15.5 mmol/L (n: 3.3-7) and 251 µmol/L (n: 45-100) from the baseline value of 9.7 mmol/L and 146 µmol/L, respectively. His white cell count was 9.1 × 109/L (n: 3.6-11.5) with an eosinophil count of 0.4 × 109/L (n: 0-0.5), and normal liver function tests. On the third day of admission, the patient developed bluish discoloration of the right toes with palpable proximal and distal pedal pulses. (Fig. 1)
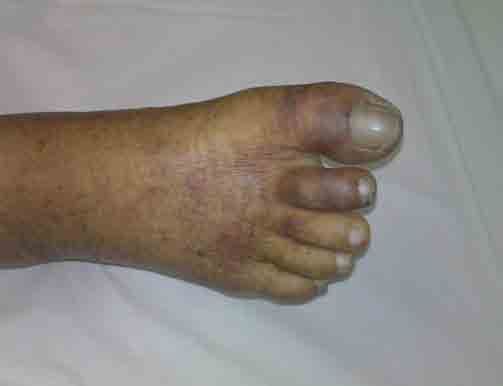
Figure 1: Bluish discoloration of the toes in a patient with acute myocardial infarction, femoral artery cannulation and thrombolysis/anticoagulation.
Questions
1. What diagnosis do the blue toes suggest in this patient?
2. What are the possible causes?
3. Which organs are commonly involved in this syndrome and what other investigations are needed?
4. What treatment options are available?
Answers
1. The blue toes suggest "Blue Toe Syndrome."
2. Blue toe syndrome results from either cholesterol crystal, thrombo-emboli, or vasculitic occlusion of small vessels.1
Cholesterol crystal embolization (CCE) from a severely atherosclerotic artery (aorta, iliac, or femoral) can occur spontaneously or from a variety of insults such as invasive vascular procedures, anticoagulation, or thrombolytic therapy, which probably remove blood clots that cover up a damaged atherosclerotic plaque leading to cholesterol-rich debris entering the bloodstream.2,3 All these factors were involved in this patient. Some of the postulated predisposing factors associated with the development of CCE are diabetes mellitus, systemic hypertension, cigarette smoking, hypercholesterolemia, age >55 years, elevated C-reactive protein, and white race.2-4
3. The skin and the kidneys are most frequently involved in blue toe syndrome.
Livedo reticularis of the lower limbs and acrocyanosis are the most common cutaneous manifestations along with raised blood eosinophil count.1,2 Histological examination is required to definitively diagnose cholesterol crystal embolization (needle-shaped cholesterol clefts); otherwise, a clinical diagnosis can be made from the characteristic presentation.2 In a large study of 1786 catheterization patients, 1.4% developed CCE, 48% had cutaneous signs, and 64% had renal insufficiency.2 Cholesterol Embolism Study (CHEST) investigators listed cutaneous manifestations (e.g., livedo reticularis, blue toe syndrome or digital gangrene) and acute renal insufficiency as criterion 1 and 2, respectively.2 The "definite" diagnosis was defined as presence of cutaneous signs with or without renal impairment, following an invasive arterial procedure and "possible" diagnosis was defined as presence of only renal dysfunction without skin manifestations 2 weeks following invasive procedure. In this patient, the diagnosis was "definite" as he had skin changes as well as renal impairment following invasive femoral sheath insertion. His ultrasound scan of leg vessels showed patent veins and arteries. Echocardiogram did not show any intracardiac thrombus. Gradually, his toe color and renal function improved.
4. Management is supportive, but few case reports in literature have noted successful treatment with corticosteroids and cyclophosphamide in patients with deteriorating renal function.1-5
According to the theory proposed by Jones and Iannaccone,6 cholesterol emboli induce intense inflammatory response with vascular changes occurring in three phases. In the first two phases, crystals cause endothelial injury, histiocytic response, and intimal proliferation. In the late phase, the crystals are entrapped by histiocytes and additional intimal proliferation, and fibrous tissue formation occurs. Based on this theory, steroids may attenuate the CCE-induced inflammatory response in the first two phases of the disease.3
In addition, anticoagulants and further vascular interventions should be avoided.3 Statins, low-density lipoprotein apheresis, and intravenous administration of the prostacyclin analogue iloprost have been reported to be useful in improving skin lesions and renal function in few patients.7-9 In a prospective study of 95 patients with CCE over a 5-year period, Scolari et al. reported end-stage renal disease and death in 24% and 38%, respectively.7 This case illustrates that clinicians should be aware of this potentially dangerous iatrogenic complication and take precautions to avoid it.
Acknowledgments
The authors reported no conflict of interest and no funding was received on this work.
|