Blunt injury to the thoracic aorta is reported in large trauma registries at a rate of 0.2–0.5%.1,2 Victims are usually males in their fourth and fifth decades of life.3–6 Motor vehicle collision (MVC) is the most commonly reported cause of injury. Other causes of injury include falls from height, pedestrians versus vehicles, and motorcycle accidents.4,5,7,8
Exsanguination secondary to aortic injuries is the second most common cause of death in trauma after traumatic brain injuries.9,10 One of the earliest reports reported 90% mortality within six hours of injury.11 Subsequent reports emphasized the high mortality associated with blunt traumatic aortic injuries (BTAIs).7,12–16 BTAIs are classified into four grades: I) intimal tears, II) intramural hematoma, III) pseudoaneurysm, and IV) complete transection.17 The aortic isthmus is the most commonly injured area.11,15 Mortality is potentially attributed to associated severe injuries to the head, chest, abdomen, and orthopedic injuries7,8,11,16,18 or presence of aortic transection (i.e., grade IV BTAI).7,19
The use of thoracic endovascular aortic repair (TEVAR) in the management of BTAI has surpassed open surgical repair in numbers over the past two decades.20 It is a safer procedure associated with less risk of death, permanent disability, and other morbidities [Figure 1].17,18,20,21
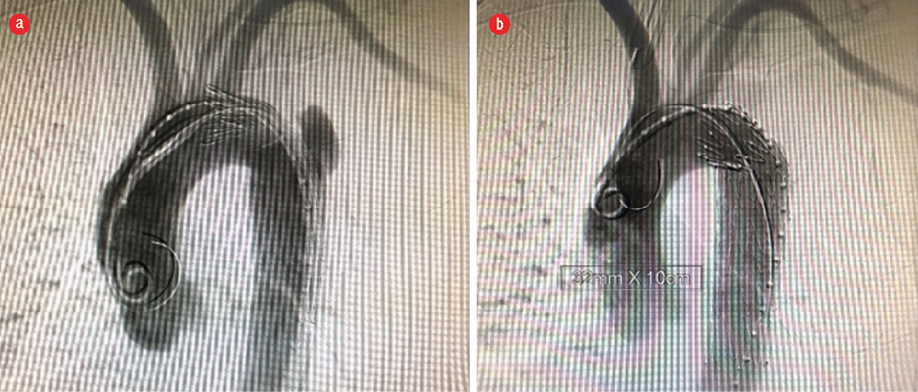
Figure 1: (a) Angiogram showing blunt traumatic aortic injury before thoracic endovascular aortic repair. (b) Completion angiogram post-thoracic endovascular aortic repair.
Methods
We conducted a retrospective analysis of a prospective multi-center registry that included 40 consecutive blunt trauma patients presenting with an image-proven diagnosis of BTAI between January 2012 and July 2017.
Ethical approval was obtained from Sultan Qaboos University Hospital, a tertiary care teaching hospital, Royal Hospital, a tertiary care hospital, and Khoula Hospital, the capital’s main trauma center. Forty consecutive patients were identified out of which four were excluded for incomplete data. The remaining 36 patients were divided based on the timing of repair into early (< 7 days) or delayed (≥ 7 days) repair. Patient’s electronic records were reviewed to obtain their demographic information, date of injury, mechanism of injury, and injury severity score (ISS). Specific aortic injury details recorded were injury grade, native aorta diameter, and distance from injury to left subclavian artery (LSA). During admission, the need for blood products transfusion, use of anti-impulse medications, anticoagulation, intensive care unit (ICU) stay, and total hospital stay were also noted. Operative details such as LSA coverage status, degree of stent graft oversizing, device access site, and technical success were also recorded. Reported complications during admission and follow-up were categorized into aorta-related and non-aorta-related (i.e., respiratory, renal, cerebrovascular, paraplegia, thromboembolic, gastrointestinal, access site-related, and others). Total follow-up time and the need for reintervention were also included in the data collection sheet. Primary endpoints included in-hospital mortality, aortic-related morbidity, non-aortic-related morbidity, and the need for reintervention.
Patients with an incidental diagnosis of BTAI beyond index trauma admission and those with incomplete data were excluded.
Data were summarized using mean, standard deviation, median, frequency, and percentage. Independent samples t-test and Mann-Whitney U-test were used to analyze parametric and non-parametric continuous variables, respectively. Chi-square test and Fisher’s exact test were used to analyze categorical variables. A p-value ≤ 0.050 was considered statistically significant. All analysis was carried out using SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
Our study subjects were young with a mean age of 33.5±14.8 and 29.9±11.0 years in the early repair and delayed repair cohorts, respectively (p = 0.447). MVC accounted for the majority of cases (82.6% and 76.9% in early and delayed repair groups, respectively, p = 0.893). Half of our patients were transferred from another facility for TEVAR (60.9% in the early repair group and 38.5% in the delayed group (p = 0.299)). The delayed repair group had a higher but not statistically significant ISS (39.4±17.8 vs. 34.9±12.3, p = 0.425). Complete demographic data is available in Table 1.
Thoracic injuries including pneumothorax, hemothorax, and lung injuries were the most commonly associated injuries in both early and delayed repair groups [Table 2]. Compared to early repair, patients who had undergone delayed repair had a higher incidence of exploratory laparotomies, but the difference was not statistically significant [Table 2].
Table 1: Demographics of patients undergoing thoracic endovascular aortic repair.
|
Sex, n (%) |
|
|
|
|
Male |
22 (95.7) |
11 (84.6) |
0.539 |
|
Female |
1 (4.3) |
2 (15.4) |
|
|
Age, mean ± SD, years |
33.5 ± 14.8 |
29.9 ± 11.0 |
0.447 |
|
Mechanism of injury, n (%) |
|
|
|
|
MVC |
19 (82.6) |
10 (76.9) |
0.893 |
|
Fall from height |
1 (4.3) |
1 (7.7) |
|
|
Pedestrian vs. vehicle |
3 (13.0) |
2 (15.4) |
|
|
Transfer, n (%) |
14 (60.9) |
5 (38.5) |
0.299 |
|
ISS, mean ± SD |
34.9 ± 12.3 |
39.4 ± 17.8 |
0.425 |
|
Aortic injury grade, n (%) |
|
|
|
|
I |
1 (4.3) |
0 (0.0) |
|
|
II |
0 (0.0) |
0 (0.0) |
|
|
III |
21 (91.3) |
13 (100) |
|
|
IV |
0 (0.0) |
1 (7.7) |
|
|
Native aortic diameter, mean ± SD, mm |
16.8 ± 5.8 |
17.6 ± 3.3 |
0.640 |
|
Distance from LSA, mean ± SD, mm |
24.3 ± 24.0 |
19.4 ± 12.3 |
0.506 |
|
Blood transfusion, n (%) |
18 (78.3) |
7 (53.8) |
0.153 |
|
Anti-impulse therapy, n (%) |
20 (87.0) |
8 (61.5) |
0.107 |
|
Anticoagulation, n (%) |
18 (78.3) |
11 (84.6) |
1.000 |
|
OT time, mean ± SD, minutes |
144.1 ± 88.1 |
124.4 ± 45.3 |
0.529 |
|
Paraplegia, n (%) |
0 (0.0) |
0 (0.0) |
|
|
Stroke, n (%) |
3 (13.0) |
1 (7.7) |
1.000 |
|
ICU stay, mean ± SD, days |
7.8 ± 6.8 |
5.3 ± 10.7 |
0.386 |
|
Total hospital stay, mean ± SD, days |
19.4 ± 15.5 |
34.0 ± 41.4 |
0.242 |
|
In-hospital mortality, n (%) |
1 (4.3) |
0 (0.0) |
1.000 |
SD: standard deviation; MVC: motor vehicle collision; ISS: injury severity score; LSA: left subclavian artery; OT: operating theater; ICU: intensive care unit.
Table 2: Associated injuries in patients undergoing thoracic endovascular aortic repair.
|
Head |
7 (30.4) |
5 (38.5) |
0.720 |
|
Lungs |
19 (82.6) |
12 (92.3) |
0.634 |
|
Ribs |
14 (60.9) |
9 (69.2) |
0.727 |
|
Other thoracic |
21 (91.3) |
8 (61.5) |
0.073 |
|
Liver |
4 (17.4) |
4 (30.8) |
0.422 |
|
Spleen |
9 (39.1) |
4 (30.8) |
0.727 |
|
Other abdominal |
9 (39.1) |
5 (38.5) |
1.000 |
|
Spinal |
7 (30.4) |
4 (30.8) |
1.000 |
|
Musculoskeletal |
15 (65.2) |
9 (69.2) |
1.000 |
|
Laparotomy |
2 (8.7) |
4 (30.8) |
0.161 |
There were four cerebrovascular accidents (CVAs) post-TEVAR. There were two symptomatic ischemic CVAs manifesting with paresis in one patient 21 days post-TEVAR and dysphasia in the other patient five days post-TEVAR, and one patient with symptomatic hemorrhagic CVA manifesting with paresis. The fourth patient presented with delirium 17 days post-TEVAR and was found to have a proximal stent migration causing non-occlusive thrombosis of major vessels [Figure 2]. Computed tomography (CT) of the head showed bilateral basal ganglia stroke [Figure 3]. He underwent aortic debranching with bilateral aortic-carotid bypass and was discharged 14 days later in good condition. Proximal stent migration was associated with a higher incidence of asymptomatic CVAs (p = 0.050). Total and partial LSA coverage was necessary for six and three patients, respectively. There was no statistically significant correlation between coverage and incidence of CVA (p = 0.220), type 1 endoleak (p = 0.466), or type 2 endoleak (p = 0.102).
Furthermore, neither the mean native aortic diameter (i.e., diameter proximal to the area of injury) nor the distance from injury to LSA affected the incidence of type 1 and 2 endoleaks (p = 0.501 and p = 0.483, respectively). There was no statistically significant difference in the incidence of aorta-related or non-aorta-related complications between our two cohorts. Our data showed that the early repair cohort had a longer but not statistically significant ICU stay (7.8±6.8 vs. 5.3±10.7, p = 0.386). Prolonged ICU stay was associated with greater likelihood to require blood transfusion (p < 0.001), incidence of respiratory complications (p = 0.010), and gastrointestinal complications (p = 0.026).
There was one recorded in-hospital mortality in our population overall in the early repair cohort. The reintervention rate was 4.3% vs. 7.7% in the early and delayed repair cohorts, respectively (p = 1.000).
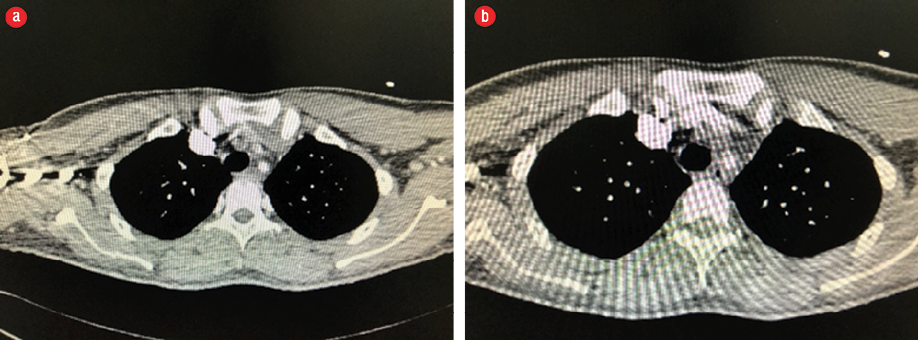
Figure 2: Computed tomography-angiogram showing (a) proximal stent graft migration and brachiocephalic thrombosis and (b) left common carotid and left subclavian thrombosis.
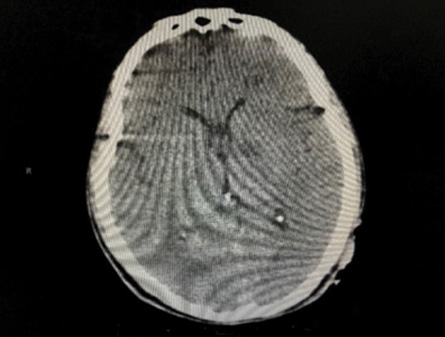
Figure 3: Brain computed tomography showing bilateral basal ganglia infarct.
Discussion
We have taken an interest in the ‘trauma epidemic’ in Oman, specifically MVC-related mortalities and morbidities due to the large burden it poses on the population. According to the 2013 World Health Organization global report on road safety, there were 30.4 recorded MVC-related mortality per 100 000 population in Oman in comparison to 11.4 and 6.8 per 100 000 in the US and Canada, respectively. Of all MVC victims, 1.4% survive with permanent disability.22
This study represents Oman’s experience with TEVAR for BTAI since its introduction to trauma care with the first repair taking place in January 2012. Our study population was comparable to previous reports in terms of young age and male predominance.3,4,17,23 In our study, native aortic diameter proximal to site of injury was significantly narrower than previously reported in other studies,17 which compounded with the young age at the time of TEVAR poses a serious question on stent graft durability.17,24
The concept of delaying management of hemodynamically normal BTAI for other immediately life-threatening injuries to be managed is evident in the literature. In 2014, the Eastern Association for the Surgery of Trauma advocated for delayed repair citing lower incidences of paraplegia and mortality.25 The 2011 Society of Vascular Surgery also offered similar recommendations in favor of prioritizing management of other life-threatening injuries and performing TEVAR before patient discharge.24 Moreover, multiple institutions cited similar results with a clear reduction in mortality.6,14 In our study, we elected to divide patients into early (< 7 days since injury) and delayed (≥ 7 days) repair cohorts.
Multiple factors contributed to the delay between injury and intervention in our population. As much as two-thirds of the early repair and more than one-third of the delayed repair patients required transfer from another hospital after diagnosis [Table 1]. Many of these patients had significant associated injuries as clearly indicated by a high mean ISS score of 34.9±12.3 and 39.4±17.8 in early and delayed repair groups, respectively. Previous studies have emphasized the impact of an initial high ISS on BTAI grade and potential survival.23,26 Moreover, thoracic aortic stent grafts are not always available off the shelf in our centers.
Anti-impulse therapy was prevalent in our population (77.8%, n = 28) overall as a bridge to TEVAR while associated injuries were being managed. Despite the positive impact of blood pressure control in lowering complications with low grade injuries (i.e., grade I intimal tears),4,23 a variable success rate is reported in preventing injury progression and exsanguination for higher grade injuries, such as pseudoaneurysms and transections (grade III and IV),4,19,27 which comprised 97.2% (n = 35) of our subjects.
The last two decades have seen a significant paradigm shift in BTAI management from traditional open repair to TEVAR.20,24,25 Endovascular management is a less morbid option.5,17,18,20,21 In our experience, there was no recorded paraplegia post-TEVAR. This is comparable to larger BTAI experiences which report a less than 1% risk.14,20 There was no statistically significant difference in the incidence of aortic or non-aortic complications between the early and delayed cohorts. Aorta-related complications were encountered in six patients (16.7%). One patient had a type 1 endoleak immediately post-TEVAR, which was managed with balloon angioplasty. A repeat CT-angiogram, on day three post-TEVAR, showed no endoleak. The second patient had both type 1 and type 2 endoleaks post-TEVAR. He underwent an unsuccessful angioembolization on post-deployment day two followed by a successful embolization on post-deployment day six. At 38 days post-deployment, a repeat CT-angiogram showed no evidence of endoleak. Two patients had type 2 endoleak. One patient had no evidence at three days post-deployment on CT angiography (CTA) while the other patient was lost to follow-up.
Furthermore, two patients had documented proximal stent graft migration on follow-up CTA. The first patient presented on post-TEVAR day 17 with headache and dizziness but no paresis. A brain CT with thoracic CTA showed bilateral parietal hypodensities and non-occlusive thrombosis of all three major vessels. He was noted to have a bovine aortic arch at deployment with LSA coverage. He subsequently underwent bilateral aortic-carotid bypass one day later and was discharged with no neurological deficits 14 days later. The second patient had CTA evidence of proximal stent graft migration and non-opacification of the LSA four months post-TEVAR but no symptoms. In a large experience published by the American Association for the Surgery of Trauma, endograft-related complications were reported in up to 20% of cases, possibly due to lack of appropriate devices for BTAI.20 Our study recorded four CVA (three in early repair and one in delayed repair, p = 1.000). Previous studies report stroke rates at 2–5% compared to our CVA rate of 11.1%.5,18 We could not find a statistically significant correlation between the incidence of CVA and injury distance from the LSA, LSA coverage, or proximal stent graft migration.
The only recorded mortality in our series was attributed to severe acute respiratory distress syndrome (ARDS) post-TEVAR. This patient underwent TEVAR the day after trauma (i.e., early repair cohort). In the ICU, he developed severe ARDS with no clear etiology eventually passing away 13 days post-TEVAR. There were no recorded aorta-related mortalities.
Conclusion
The short-term outcomes for TEVAR of BTAI continue to show its feasibility in managing BTAI in severely injured patients. There was no clear statistical significance in mortality and morbidity comparing early repair versus delayed repair. However, our experience is based on a small sample size and short median follow-up but provides a good platform for further analysis.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Forman MJ, Mirvis SE, Hollander DS. Blunt thoracic aortic injuries: CT characterisation and treatment outcomes of minor injury. Eur Radiol 2013 Nov;23(11):2988-2995.
- 2. Dua A, Desai SS, Kuy S, Patel B, Dua A, Desai PJ, et al. Predicting outcomes using the National Trauma Data Bank: optimum management of traumatic blunt carotid and blunt thoracic injury. Perspect Vasc Surg Endovasc Ther 2012 Sep;24(3):123-127.
- 3. Zipfel B, Chiesa R, Kahlberg A, Marone EM, Rousseau H, Kaskarelis I, et al; RESTORE Investigators. Endovascular repair of traumatic thoracic aortic injury: final results from the relay endovascular registry for thoracic disease. Ann Thorac Surg 2014 Mar;97(3):774-780.
- 4. Gunn ML, Lehnert BE, Lungren RS, Narparla CB, Mitsumori L, Gross JA, et al. Minimal aortic injury of the thoracic aorta: imaging appearances and outcome. Emerg Radiol 2014 Jun;21(3):227-233.
- 5. Azizzadeh A, Charlton-Ouw KM, Chen Z, Rahbar MH, Estrera AL, Amer H, et al. An outcome analysis of endovascular versus open repair of blunt traumatic aortic injuries. J Vasc Surg 2013 Jan;57(1):108-114; discussion 115.
- 6. Symbas PN, Sherman AJ, Silver JM, Symbas JD, Lackey JJ. Traumatic rupture of the aorta: immediate or delayed repair? Ann Surg 2002 Jun;235(6):796-802.
- 7. Franzen D, Genoni M. Analysis of risk factors for death after blunt traumatic rupture of the thoracic aorta. Emerg Med J 2015 Feb;32(2):124-129.
- 8. Di Eusanio M, Folesani G, Berretta P, Petridis FD, Pantaleo A, Russo V, et al. Delayed management of blunt traumatic aortic injury: open surgical versus endovascular repair. Ann Thorac Surg 2013 May;95(5):1591-1597.
- 9. Clancy TV, Gary Maxwell J, Covington DL, Brinker CC, Blackman D. A statewide analysis of level I and II trauma centers for patients with major injuries. J Trauma 2001 Aug;51(2):346-351.
- 10. Pfeifer R, Tarkin IS, Rocos B, Pape H-C. Patterns of mortality and causes of death in polytrauma patients–has anything changed? Injury 2009 Sep;40(9):907-911.
- 11. Parmley LF, Mattingly TW, Manion WC, Jahnke EJ Jr. Nonpenetrating traumatic injury of the aorta. Circulation 1958 Jun;17(6):1086-1101.
- 12. Mwipatayi BP, Boyle A, Collin M, Papineau J-L, Vijayan V. Trend of management of traumatic thoracic aortic injuries in a single center. Vascular 2014 Apr;22(2):134-141.
- 13. Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg 2012 Jan;55(1):47-54.
- 14. Estrera AL, Gochnour DC, Azizzadeh A, Miller CC III, Coogan S, Charlton-Ouw K, et al. Progress in the treatment of blunt thoracic aortic injury: 12-year single-institution experience. Ann Thorac Surg 2010 Jul;90(1):64-71.
- 15. Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, et al. Blunt thoracic aortic injuries: an autopsy study. J Trauma 2011 Jan;70(1):197-202.
- 16. Duwayri Y, Abbas J, Cerilli G, Chan E, Nazzal M. Outcome after thoracic aortic injury: experience in a level-1 trauma center. Ann Vasc Surg 2008 May-Jun;22(3):309-313.
- 17. Azizzadeh A, Ray HM, Dubose JJ, Charlton-Ouw KM, Miller CC, Coogan SM, et al. Outcomes of endovascular repair for patients with blunt traumatic aortic injury. J Trauma Acute Care Surg 2014 Feb;76(2):510-516.
- 18. Murad MH, Rizvi AZ, Malgor R, Carey J, Alkatib AA, Erwin PJ, et al. Comparative effectiveness of the treatments for thoracic aortic transection [corrected]. J Vasc Surg 2011 Jan;53(1):193-199. e1-21.
- 19. Riesenman PJ, Brooks JD, Farber MA. Acute blunt traumatic injury to the descending thoracic aorta. J Vasc Surg 2012 Nov;56(5):1274-1280.
- 20. Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Diagnosis and treatment of blunt thoracic aortic injuries: changing perspectives. J Trauma 2008 Jun;64(6):1415-1418, discussion 1418-1419.
- 21. Dake MD, White RA, Diethrich EB, Greenberg RK, Criado FJ, Bavaria JE, et al; Society for Vascular Surgery Outcomes Committee. Report on endograft management of traumatic thoracic aortic transections at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg 2011 Apr;53(4):1091-1096.
- 22. World Health Organization. World Health Organization, editors. Global status report on road safety 2013: supporting a decade of action. Geneva, Switzerland: World Health Organization; 2013. p.303.
- 23. Rabin J, DuBose J, Sliker CW, O’Connor JV, Scalea TM, Griffith BP. Parameters for successful nonoperative management of traumatic aortic injury. J Thorac Cardiovasc Surg 2014 Jan;147(1):143-149.
- 24. Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg 2011 Jan;53(1):187-192.
- 25. Fox N, Schwartz D, Salazar JH, Haut ER, Dahm P, Black JH, et al. Evaluation and management of blunt traumatic aortic injury: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015 Jan;78(1):136-146.
- 26. El-Beheiry MH, Kidane B, Zehr M, Vogt K, Parry NG, Malthaner R, et al. Predictors of discharge home after blunt traumatic thoracic aortic injury. Ann Vasc Surg 2016 Jan;30:192-197.
- 27. Mosquera VX, Marini M, Lopez-Perez JM, Muñiz-Garcia J, Herrera JM, Cao I, et al. Role of conservative management in traumatic aortic injury: comparison of long-term results of conservative, surgical, and endovascular treatment. J Thorac Cardiovasc Surg 2011 Sep;142(3):614-621.