|
Abstract
Sarcoidosis is a common multi-system disease characterized histolopathologically by the formation of non-caseating granulomas in the affected tissues. The oral involvement of sarcoidosis is relatively rare with less than 70 reported cases in literature while an oral lesion as the initial presenting sign is even less common. Oral lesions of sarcoidosis may mimic the lesions of other serious systemic diseases including Crohn’s disease and tuberculosis as well as lesions localized to the orofacial region such as orofacial granulomatosis. This report presents a case of non-progressive sarcoidosis where the initial presenting symptom was a lesion in the buccal vestibule attached to the gingivae. A brief review of the pathology and clinical features is also presented.
Keywords: Sarcoidosis; Non-caseating granulomas; Orofacial granulomatosis.
Introduction
Sarcoidosis is a common multi-system granulomatous disease that is characterized by the formation of non-caseating epithelioid cell granulomas in various tissues and organs. The first case of sarcoidosis described in literature was by Hutchinson in 1875.1 The disease mainly affects the lungs, lymph nodes,2,3 liver, and spleen, and less frequently the eyes, bones and skin.1,4 In the head and neck region, the most commonly affected structures are the salivary glands especially the parotid gland where it occurs in 6% of sarcoidosis patients,3,5 and the cervical lymph nodes.2 Oral involvement of sarcoidosis is relatively rare and usually the buccal mucosa, lips, gingivae, tongue and palate are the affected sites.1,2,4 The first reported oral lesion of sacroidosis was reported by Schroff in 1942.1,6 Most recent reviews of the literature describe around 70 case reports of sarcoidosis with oral involvement and after excluding the parotid gland and cervical lymph nodes, the oral involvement becomes a very rare finding.1,3
The incidence of sarcoidosis is variable worldwide. In the USA, there is significant racial variation in the incidence rate, with 10-14 cases per 100,000 for Caucasians and 35.5-64 cases per 100,000 for African-Americans.1,7 Sarcoidosis is more frequent among Africans than Caucasians as demonstrated by various studies.8 There is a slightly higher incidence in females.7,8
Case Report
An 84-year-old female was referred to an oral and maxillofacial surgeon for the investigation and management of a longstanding lump in the left mandibular buccal vestibule. The lump was attached to the buccal gingivae adjacent to the left mandibular first premolar and running over the left mental nerve. There was no change in sensation of the lower lip but the premolar was sensitive to vertical and lateral pressure. Radiographs show a periapical radiolucency with extruded endodontic material extending into the radiolucency.
Medically, she had medication controlled hypertension with no history of cardiac disease. She was also on prophylactic aspirin. She had moderate shortness of breath on exertion but required no medication for this.
Orally, she had a long term history of dry mouth. At age 77, the left parotid duct was clinically obstructed with no evidence of parotid enlargement and a consultant oral and maxillofacial surgeon had recommended no action. The buccal swelling had been noted by the patient and her general dentist for many years. It had not changed over time. The left mandibular premolar had been root-filled partly in response to the lump but had no effect on it. When the tooth became mildly symptomatic she was referred to an endodontist who indicated that the treatment choices were to do endodontic retreatment, to have the lump removed and the tooth apicectomied, or to have the lump removed and the tooth extracted. The patient selected to have removal of the lump and an apicectomy and was referred to an oral and maxillofacial surgeon for the procedure.
Under local anesthesia, the lump was dissected off the left mental nerve. An apical seal was obtained by minor root sectioning. The specimen was submitted for histopathology. Post-operatively, the wound healed well. The patient, however, had a small area of sensory loss consistent with the nerve fiber in the proximity to the dissection. Based on the findings, the buccal lump in this case was non-odontogenic in origin.
Histopathological examination by the hematoxylin and eosin (H&E) staining demonstrated non-caseating epithelioid cell granulomas, typical of granulomatous conditions (Figs. 1-3). Multinucleated giant cells were evident throughout the granuloma (Fig. 3). Based on the histological findings in the H&E stained tissues, a number of special investigations were undertaken. Ziehl-Neelsen staining did not demonstrate evidence of acid fast bacilli thus, tuberculosis was excluded. Serum angiotensin-converting enzyme (S-ACE) blood test was organized. The result of 153 u/L is elevated above the normal. Accordingly, further investigations including a chest X-ray and CT were arranged. Although these showed no evidence of sarcoid lung involvement, there was mild bronchiectasis and evidence of small airway disease in the lungs. Eye examination was normal.
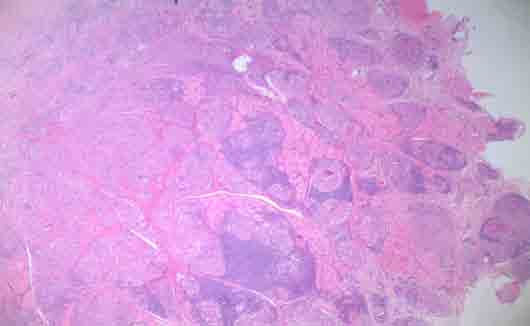
Figure 1: Photomicrograph of H&E stained buccal mucosa shows the formation of multiple granulomas without caseation (magnification × 4).
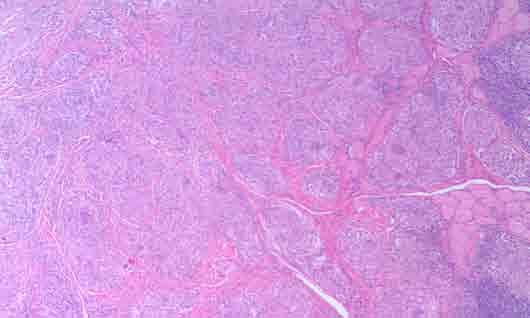
Figure 2: Photomicrograph of H&E stained buccal mucosa and the granulomas within fibrous connective tissues and adjacent muscle tissues (magnification × 10).
Further investigations were not indicated or performed. Diagnosis of nonprogressive sarcoidosis was suggested. At 18- month review, the tooth was asymptomatic and the lump has not recurred. The lip numbness was still present but reduced. Annual medical checkup showed ongoing small airway disease and the S-ACE level remains elevated.
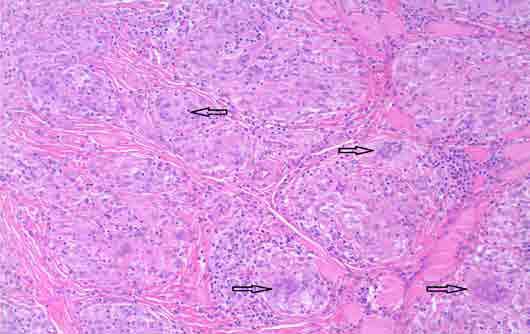
Figure 3: Photomicrograph shows the multinucleated giant cells [arrows] (magnification × 20).
Discussion
Systemic sarcoidosis is a common multi-system disease characterized by the formation of non-caseating granulomas in the affected tissues. It may manifest as nonspecific symptoms such as fever, malaise, weight loss, and fatigue in one third of the patients.1,9 Lesions of sarcoidosis manifest in the skin in the form of lupus pernio or in the form of erythema nodosum.10 The histology of lupus perni shows the typical non-caseating granulomas; however, these granulomas are not detected in the lesions of erythema nodosum.10 Eye involvement presents as ocular iritis, iridocylitis associated with dryness and photophobia. Liver and spleen might be enlarged due to the formation of granulomas. Functions of liver and spleen may be compromised. Bone marrow involvement is also common but to a lesser extent in the jaw bones.1,11
The etiology of sarcoidosis is not well understood. There is a genetic predisposition suggested by the presence of familial clusters in sarcoidosis.12 There is thought to be an immunological response triggered by unknown factors manifesting as sarcoidosis and has higher prevalence in genetically susceptible individuals.8 Some viral and bacterial infections such as mycobacteria, Epstein-Barr virus (EBV) and human herpesvirus-8 have also been attributed to be causative factors of sarcoidosis.9 Links between sarcoidosis and some external environmental factors and occupational exposures such as mold and material used in pesticides have also been demonstrated in some studies.13 The mechanism by which sarcoidosis develops is a form of immunological response to one or more of these factors which lead to the formation of the non-caseating granulomas. This immunological response of type 1 T-helper cells is a result of deposition of antigenic material in the affected tissues, which is presented to T-lymphocytes by the macrophages or dendritic cells. T-helper cells produce chemokines and cytokines which bring about amplification of cellular immune response and migration of mononuclear phagocytes and other inflammatory cells into the tissues.8,9,13
There are no specific tests for sarcoidosis. Diagnosis of sarcoidosis is mainly based on clinical characteristics,1,3,14 as well as chest X-ray and exclusion of other non-caseating granulomas forming conditions.14 The majority of cases have lung involvement (90%) in the form of pulmonary infiltration and involvement of thoracic lymph nodes.8 Medical and radiographical examination usually reveals pulmonary hypofunction and chest X-ray shows typical bilateral hilar lymphadenopathy. Hematological and biochemical tests may show elevated erythrocyte sedimentation rate, anemia, lymphocytopenia, increased liver enzymes, hypercalcemia and increased serum angiotensin enzyme (S-ACE).1,9 S-ACE is positive in 80% cases of sarcoidosis and may be false negative in some cases with other systemic diseases.15
The diagnosis of sarcoidosis is usually supported by the histological appearance of the non-caseating epitheloid cell granulomas in affected organs or tissues.16 Histopathological examination shows the typical non-caseating granulomas which contain epithelioid macrophages surrounded by lymphocytes. Multinucleated giant cells form due to the fusion of these mononuclear cells. Schaumann bodies which contain calcium oxalate crystals are found in 48% to 88% of the patients. Asteroid or satellite bodies are interesting occasional findings seen in less than 2% and 9% of the cases.1,16
The use of Kveim-Siltzbach test is historical because of its low sensitivity and cross infection considerations.17,18 This test involved the injection of an extract from the spleen of a known sarcoidosis case into the skin of the suspected case. Histopathology of formed skin nodule (after 4-6 weeks) in positive cases confirms the diagnosis.1,17
Some syndromes have been described to involve other clinical features beside the sarcoid lesion. Heerfordt syndrome is characterized by parotid gland enlargement, uveitis, and facial palsy.3 Löfgren’s syndrome is characterized by bilateral hilar lymphadenopathy and erythema nodosum.10
The presented case demonstrates that sarcoidosis may, in rare cases, manifest initially as an oral lesion. From all orally involved sarcoidosis, in one-third of these cases the oral lesion will be the first clinical presentation.1,3 The previously published data on oral lesions of sarcoidosis show variable locations in the oral cavity. The jaw bone represent the most common location for oral involvement with 29.6% occurrence rate.3 It usually presents as lytic lesions and loose teeth.1,3 Others, but less frequent locations for oral involvement, are the buccal and gingival mucosa. The lips, tongue, and palate are least common sites for oral involvement,1,3 while 42% of case have multiple lesions at the same time.3
Granulomatous lesions of the oral cavity present histopathologically as non-caseating epithelioid cell granulomas. This typical histological appearance is common in Crohn’s disease, sarcoidosis, and orofacial granulomatosis (OFG). Tuberculosis granulomas are usually associated with necrosis. OFG is the clinical term used to describe the presence of non-caseating granulomas in the oral cavity and perioral region with the absence of any systemic condition.19 It is usually characterized by a number of features such as asymptomatic labial swelling, cobblestone appearance of the buccal mucosa, gingival enlargement, mucosal ulcerations, and mucosal tags.19
Conclusion
Oral granulomatous lesions can occur as isolated lesions as in orofacial granulomatosis but they should also be considered as manifestation of more complex diseases such as tuberculosis, Crohn’s disease or sarcoidosis. Although it is very rare, oral lesions of sarcoidosis may be the first presenting symptom. The indistinguishable histological features of these conditions demand that oral surgeons and pathologists to investigate all possible underlying systemic diseases.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work. |