Complete blood count is a commonly performed test in the neonatal intensive care unit. The results have various clinical ramifications (e.g., neutropenia would be prone to sepsis, candidiasis, etc).1 However, interpreting counts is more difficult in children, and particularly in neonates, owing to the scarcity of normative data.1
The landmark publication by Manroe et al,2 from the University of Texas South Western Medical School at Dallas, USA, published in 1979, established a method for determining whether a neonate’s neutrophil count should be considered neutrophilic, normal, or neutropenic. In 1994, the same group led by Mouzinho et al,3 published new information on neutrophil counts in very low birth weight neonates. The pioneering observations of this group in defining the normal ranges for blood neutrophil counts of term and preterm neonates, famous as Manroe and Mouzinho’s charts, are widely used reference data in the field of neonatal hematology.4
The reference ranges obtained from western populations have been used in developing countries owing to lack of indigenous normative data. However, neutrophil count patterns may be affected by ethnicity, gender and environmental factors, altitude, maternal hypertension, and maternal fever before delivery.5–10 Hence, routine application of western data to Indian context is questionable. Furthermore, there could be variations within the umbrella term ‘pediatric’ or ‘neonatal’ reference ranges. Hematologic values in neonates differ significantly from those in older children. Neonatal results may show quantitative and qualitative differences as a reflection of the developmental changes during fetal hematopoiesis, and values at birth may vary with gestational age.5 As an added confounding factor, Schmutz et al,4 revisited the Manroe and Mouzinho's charts and suggested a higher value for the upper limit of neutrophil counts than indicated in the traditional charts. This was attributed to the use of modern technology for blood count estimation and variations in altitude.
In the face of all these sources of variation, it is imperative that blood count data generated by studying newborn babies in an Indian setting should be used in compiling normative data for Indian newborns. Such data could then be used as reference ranges for interpreting neutrophil counts in Indo-Asian newborns. Therefore, we conducted an observational study to fulfill this objective.
Methods
We conducted an observational study of newborns admitted to the neonatal unit of the department of pediatric medicine in a large tertiary care teaching hospital in Kolkata, India over 14 months (May 2016 to June 2017). The institutional ethics committee approved the study protocol and written informed consent was obtained from either parent or accompanying grandparent of the recruited child.
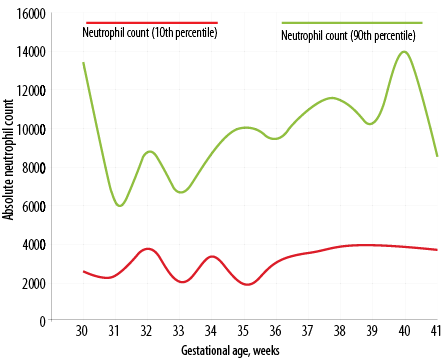
Figure 1: Day three neutrophil count percentile chart in Indian newborns as per gestational age.
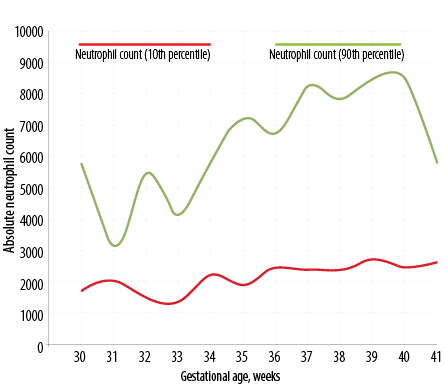
Figure 2: Day five neutrophil count percentile chart in Indian newborns as per gestational age.
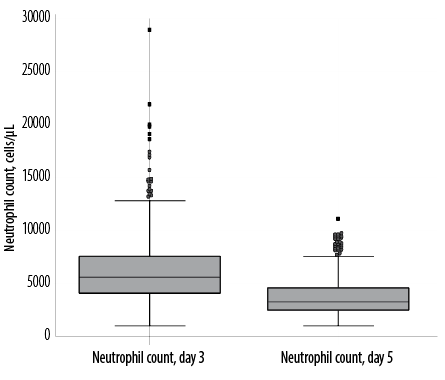
Figure 3: Box and whisker plots of neutrophil counts in the whole study cohort (n = 420).

Figure 4: Scatter plot to show correlation (correlation coefficient rho 0.871) between absolute neutrophil counts in newborns on day three and five.
Healthy newborns of either gender were recruited through purposive sampling to ensure at least 30 newborns represented every week between the gestational ages of 30 to 41 weeks. Gestational age was estimated by first trimester dating scan or, in the absence of that, as per last menstrual period (LMP) provided mother’s cycles were regular and corroborated postnatally by New Ballard Score (NBS). If there was a discrepancy exceeding two weeks between gestation age calculated using LMP and by NBS, the latter was followed. The antenatal record was checked to exclude babies with a history of intrauterine growth retardation and babies born to mothers with a history of maternal fever. Birth records were also considered to ensure that only newborns with a five-minute Apgar score > 7 were included. Sick newborns requiring mechanical ventilator support for more than 24 hours, or taking antibiotics or inotropes were excluded.
On the third and fifth postnatal day, 2 mL of venous blood were collected from each newborn into EDTA tubes. The collection was done in the daytime from a peripheral vein using a disposable 24 gauge (0.55 mm × 25 mm) hypodermic needle (Becton Dickinson, Haryana India Pvt Ltd). An automated cell counter system Sysmex XN-1000TM (Sysmex, Kobe, Japan) was used to analyze the blood samples. If the newborn became sick between the third and fifth day of life, the second blood sample was not collected, and data of these subjects were excluded.
No formal sample size calculation was done for this study, but an effort was made to include at least 30 babies per gestational week between 30 to 41 weeks. The raw data were entered into a Microsoft Excel spreadsheet and subsequently analyzed by Statistica version 6 (Tulsa, Oklahoma: StatSoft Inc., 2001) and MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software 2011) software. Birth weight and neutrophil count data were skewed by Kolmogorov-Smirnov goodness-of-fit test. Normative data summary, therefore, includes the median, percentile values, and mean±standard deviation. Counts were compared between day three and day five by Wilcoxon matched pairs signed rank test, and between genders by Mann-Whitney U test. A p-value < 0.050 was considered statistically significant. Associations between counts were quantified by calculating Spearman’s rank correlation coefficients.
Results
Of the 434 newborns recruited in the study, complete data were available for analysis from 420. Table 1 depicts the descriptive summary stratified by gender for the whole cohort. There were no statistically significant differences in neutrophil counts between male and female newborns (Mann-Whitney U test). The mean neutrophil count on day three was 6295.0±3423.0, 6390.0±3790.0, and 6208.0±3053.0 for the whole cohort, males, and females, respectively, and on day five was 3836.0±1914.0, 3807.0±1980.0, and 3837.0±1856.0 for the whole cohort, males, and females, respectively.
Figure 1 depicts the dispersion of neutrophil counts in the whole study cohort. The distributions are obviously skewed, and there are several outlier values. With neutrophil count, there is clustering of values below 8000 cells/μL on day three and below 5000 cells/μL on day five. The dispersions were wider on day three, and the median and interquartile range (IQR) values were lower on day five. The decline from day three was statistically significant (p < 0.001) for neutrophil counts (Wilcoxon matched pairs signed rank test). Table 2 shows the neutrophil count in newborns as per gestational age from 30 to 41 weeks (n = 420). The percentile chart of neutrophil generated are graphically depicted in Figure 1 (day 3) and Figure 2 (day 5). The figures can be used for checking normality of absolute neutrophil count (ANC) in neonatology departments, especially in India.
We observed skewed distributions of neutrophil counts in the whole study cohort with the median value on day five significantly lower (p < 0.001) when compared to day three [Figure 3].
Figure 4 depicts the association between neutrophil counts on day three and day five. Although values decline on day five, there is a strong association between the two sets of values (Spearman’s rank correlation coefficient rho 0.871, p < 0.050).
Table 1: Summary profile of neutrophil counts in healthy Indian newborns.
|
|
|
|
|
|
|
Range |
840–3900 |
840–3860 |
850–3900 |
0.164 |
|
Mean ± SD |
2000.0 ± 692.3 |
2054.0 ± 716.2 |
1950.0 ± 667.4 |
|
|
Median (IQR) |
1853 (1400–2604) |
1975 (1400–2670) |
1795 (1400–2590) |
|
|
10th to 90th percentile |
1195–2958 |
1200–3100 |
1165–2800 |
|
|
ANC, cells/μL – day 3 |
|
|
|
|
|
Range |
1000–28900 |
1000–28900 |
1270–20000 |
0.936 |
|
Mean ± SD |
6295.0 ± 3423.0 |
6390.0 ± 3790.0 |
6208.0 ± 3053.0 |
|
|
Median (IQR) |
5600 (4100–7600) |
5800 (3900–7600) |
5600 (4300–7600) |
|
|
10th to 90th percentile |
3000–10150 |
2400–10200 |
3300–10100 |
|
|
ANC, cells/μL – day 5 |
|
|
|
|
|
Range |
1010–11100 |
1080–11100 |
1010–9700 |
0.430 |
|
Mean ± SD |
3836.0 ± 1914.0 |
3807.0 ± 1980.0 |
3837.0 ± 1856.0 |
|
|
Median (IQR) |
3275 (2530–4580) |
3200 (2400–4600) |
3400 (2600–4500) |
|
ANC: absolute neutrophil count; IQR: interquartile range; SD: standard deviation.
Table 2: Normative data of absolute neutrophil count in Indian newborns as per gestational age (n = 420).
|
30 |
37 |
D3 |
6241.0 ± 4018.0 |
2600 |
3600 |
5100 |
8100 |
13400 |
|
D5 |
3342.0 ± 1545.0 |
1600 |
2100 |
2800 |
4300 |
5700 |
|
31 |
31 |
D3 |
4161.0 ± 2345.0 |
2400 |
3100 |
3600 |
4700 |
6100 |
|
D5 |
2649.0 ± 939.0 |
1890 |
2100 |
2400 |
3100 |
3200 |
|
32 |
32 |
D3 |
6372.0 ± 3995.0 |
3800 |
4300 |
5400 |
6950 |
8800 |
|
D5 |
3371.0 ± 1676.0 |
1400 |
2400 |
3200 |
3600 |
5400 |
|
33 |
31 |
D3 |
4550.0 ± 2256.0 |
2000 |
3200 |
4300 |
5600 |
6700 |
|
D5 |
2829.0 ± 1393.0 |
1300 |
1800 |
2760 |
3200 |
4100 |
|
34 |
30 |
D3 |
6857.0 ± 4650.0 |
3400 |
4600 |
6250 |
8400 |
8850 |
|
D5 |
3807.0 ± 1613.0 |
2100 |
2700 |
3300 |
5300 |
5850 |
|
35 |
39 |
D3 |
5911.0 ± 3639.0 |
1900 |
3500 |
5600 |
7200 |
10100 |
|
D5 |
3777.0 ± 2046.0 |
1780 |
2300 |
3200 |
4500 |
7200 |
|
36 |
50 |
D3 |
6390.0 ± 2641.0 |
3200 |
4600 |
6400 |
7600 |
9450 |
|
D5 |
4054.0 ± 1879.0 |
2350 |
2700 |
3475 |
5000 |
6750 |
|
37 |
44 |
D3 |
7116.0 ± 3200.0 |
3600 |
4550 |
6700 |
9150 |
11100 |
|
D5 |
4620.0 ± 2317.0 |
2300 |
2950 |
3950 |
6000 |
8200 |
|
38 |
37 |
D3 |
7863.0 ± 3257.0 |
3900 |
5600 |
7600 |
9100 |
11500 |
|
D5 |
4853.0 ± 2140.0 |
2300 |
3400 |
4312 |
6300 |
7800 |
|
39 |
30 |
D3 |
6802.0 ± 3122.0 |
3950 |
5600 |
6400 |
7000 |
10350 |
|
D5 |
4357.0 ± 2026.0 |
2600 |
3200 |
3800 |
4560 |
8450 |
|
40 |
29 |
D3 |
7182.0 ± 3574.0 |
3900 |
5600 |
6000 |
7700 |
13900 |
|
D5 |
4312.0 ± 2222.0 |
2360 |
2700 |
3600 |
5500 |
8400 |
|
D3 |
5562.0 ± 2368.0 |
3685 |
4390 |
5030 |
6300 |
8500 |
SD: standard deviation.
Discussion
We have quantified normative data of blood counts stratified by gestational age from 420 newborns. Neutrophil counts declined significantly from day three to five while maintaining good correlation. The values indicate that the ANC in healthy term Indian newborns is higher than indicated in Manroe and Mouzinho’s charts. Further, in preterm babies, neutrophil count does not fall as low as 1500 cells/µL.
In our study, we focused on determining gestation-wise ANC in our population context. The high neutrophil count at birth mostly arises from bone marrow mobilization of the pre-existing neutrophil pool owing to stress during birth.6 Beyond 48 hours of life most neonates show a decline of total leukocyte count and differential count with an almost equal proportion of neutrophils.7 Therefore, we decided to do the first blood sampling on day three. Repeat blood sampling was also done on day five as we presumed the count remains stable from then onwards. Hematologic parameters differ from different samples (i.e., cord blood, arterial blood, or venous blood). Neutrophil counts are better reflected in peripheral venous blood, which was used in our study.8
The Manroe et al,2 publication documented the expected range of neutrophil concentrations during the first 60 hours after delivery using data obtained from 108 term neonates hospitalized between 1974 and 1976. In 1994, Mouzinho et al,3 published new information on neutrophil counts from healthy, very low birth weight neonates clearly showing a difference in neutrophil dynamics among term and preterm babies. According to their study, ANC ranged from 500 to 2200 cells/µL 18 to 20 hours after birth.3 Values between 61 hours and 28 days had upper and lower boundaries of 6000 and 1100 cells/µL, respectively, unlike our results. Possible explanations for the difference included the timing of blood sampling (third day), ethnicity of the population studied, and the use of modern equipment in the index study. These boundaries of ANC were substantially beneath the low boundaries of the counts in larger, term newborns as reported earlier.2 Thus, if gestational age is not considered, a vast majority of premature newborns will be considered neutropenic. Therefore, reference ranges for newborns need to be matched to gestational age. Other perinatal factors that may alter neutrophil dynamics include maternal hypertension, maternal fever before delivery, and mode of delivery.9,10
Studies in the early 1990s suggested that at high altitude a much higher upper limit of neutrophil count occurs.11,12 Hence, at high altitude, Manroe and Mouzinho charts are inappropriate to diagnose neutrophilia or neutropenia. Relatively recently, Schmutz et al,4 described the expected ranges for ANC over the first 10 days of life among neonates aged 23 to 42 weeks gestation at high altitude taking advantage of modern methods of neutrophil quantification (electronic cell counter). Their findings are more in accordance with those reported by Carballo et al, and Maynard et al.11,12 Mean ANC was 7.7±3.0 × 109/L in term babies from rural Sindh in samples from cord blood, which is somewhat comparable to our result.13 Whereas another similar study from Iraq showed the mean white cell count of cord blood in term neonates was 10.12±2.8 × 109/L (range 3.1–21.6), unlike our result in term babies.14
Some studies have reported sex differences in ANC in neonates.15 A review study suggested that newborn females have neutrophil counts averaging 2000 cells/µL higher than males.10 However, we did not detect any sex differences in neutrophil counts on day three or five.
Studies on the distribution of hematological values in newborns are limited in the Indian context. A study conducted in Chandigarh over 25 years ago investigated routine hematological parameters in 240 term normal neonates – 40 neonates in the first week of life and 49 infants between three and six months old.16 The authors reported a wide variation in total and differential leukocyte counts and highlighted the difficulty in interpreting white cell counts in the newborn period. A more recent study from Chennai analyzed umbilical cord blood at birth from 120 full-term newborns of normal birth weight born out of uneventful pregnancy to mothers aged between 21 to 45 years with hemoglobin above 10 g/dL.17 They reported ANC of 5700±900 cells/µL, which is comparable to our study without any sex differences.
Our study was limited by its small sample size, use of hospital-based data, and lack of serial neutrophil count from day one to day seven. However, it is the first study of gestation-wise reference ranges of neutrophil counts from an Indo-Asian context. Thus, the gestation-wise reference ranges for neutrophil counts in the Indo-Asian context we have produced have the potential to be used as a standard for comparing counts for the precision of diagnosis of neonatal sepsis with a long-term goal of reducing neonatal mortality.
Conclusion
Normative values of neutrophil counts in Indo-Asia newborns vary from those stated in the standard chart framed by Manroe and Mouzniho for western populations. Our study has established reference ranges of neutrophil count stratified by gestational age for the Indian population, which can help in the precise diagnosis of neonatal sepsis.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Maheshwari A. Neutropenia in the newborn. Curr Opin Hematol 2014 Jan;21(1):43-49.
- 2. Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr 1979 Jul;95(1):89-98.
- 3. Mouzinho A, Rosenfeld CR, Sánchez PJ, Risser R. Revised reference ranges for circulating neutrophils in very-low-birth-weight neonates. Pediatrics 1994 Jul;94(1):76-82.
- 4. Schmutz N, Henry E, Jopling J, Christensen RD. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J Perinatol 2008 Apr;28(4):275-281.
- 5. Forestier F, Daffos F, Catherine N, Renard M, Andreux JP. Developmental hematopoiesis in normal human fetal blood. Blood 1991 Jun;77(11):2360-2363.
- 6. Engle WA, McGuire WA, Schreiner RL, Yu PL. Neutrophil storage pool depletion in neonates with sepsis and neutropenia. J Pediatr 1988 Oct;113(4):747-749.
- 7. Kurtul BE, Kabatas EU, Zenciroglu A, Ozer PA, Ertugrul GT, Beken S, et al. Serum neutrophil-to-lymphocyte ratio in retinopathy of prematurity. J AAPOS 2015 Aug;19(4):327-331.
- 8. Rotshenker-Olshinka K, Shinwell ES, Juster-Reicher A, Rosin I, Flidel-Rimon O. Comparison of hematologic indices and markers of infection in umbilical cord and neonatal blood. J Matern Fetal Neonatal Med 2014 Apr;27(6):625-628.
- 9. Proytcheva MA. Issues in neonatal cellular analysis. Am J Clin Pathol 2009 Apr;131(4):560-573.
- 10. Jacob EA. Hematological differences in newborn and aging: a review study. Hematol Transfusion Int J 2016;3(3):00067.
- 11. Carballo C, Foucar K, Swanson P, Papile LA, Watterberg KL. Effect of high altitude on neutrophil counts in newborn infants. J Pediatr 1991 Sep;119(3):464-466.
- 12. Maynard EC, Reed C, Kircher T. Neutrophil counts in newborn infants at high altitude. J Pediatr 1993 Jun;122(6):990-991.
- 13. Pasha W, Ali W, Khattak AL, Ahmed N, Idris M, Nayyer ZA. Reference haematological values for full term healthy newborns from rural Sindh, Pakistan. J Ayub Med Coll Abbottabad 2015 Apr-Jun;27(2):375-377.
- 14. Al-Marzoki JM, Al-Maaroof ZW, Kadhum AH. Determination of reference ranges for full blood count parameters in neonatal cord plasma in Hilla, Babil, Iraq. J Blood Med 2012;3:113-118.
- 15. Katsares V, Paparidis Z, Nikolaidou E, Karvounidou I, Ardelean KA, Drossas N, et al. Reference ranges for umbilical cord blood hematological values. Lab Med 2009;40(7):437-439.
- 16. Marwaha N, Marwaha RK, Narang A, Thusu K, Garewal G, Bhakoo ON. Routine hematological values in term newborns. Indian Pediatr 1992 Sep;29(9):1095-1099.
- 17. Suman FR, Raj RS, Priyathersini N, Rajendran R, Rajendran R, Ramadoss U. Biological reference interval for hematological profile of umbilical cord blood: a study conducted at a tertiary care centre in south India. J Clin Diagn Res 2015 Oct;9(10):SC07-SC09.