A 33-year-old gravida III (para 1, abortion 1, living 1) woman with poor antenatal care delivered a male infant by spontaneous vaginal delivery. The newborn had a live, beating heart protruding through an anterior chest wall defect [Figure 1 and 2]. The baby was noted to be cyanosed with a post-ductal saturation of 70–75% at 30 minutes of life. No other midline defects were identified. Referral to a tertiary facility was immediately arranged for further workup and management. Although vigorous at birth, the baby expired during transport.
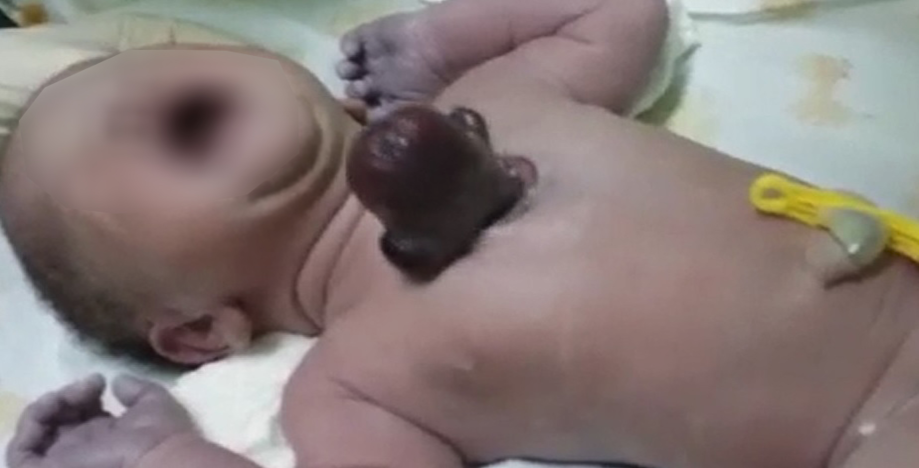
Figure 1: A cyanosed, term neonate with an isolated extra-thoracic chest mass.
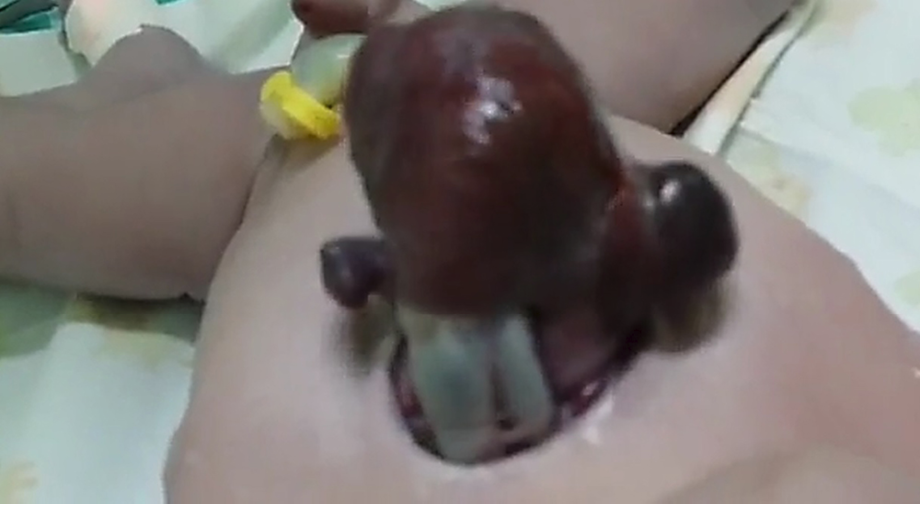
Figure 2: The ‘mass’ eviscerating through the anterior chest wall with part of great vessels.
Question
- What is the most likely clinical diagnosis?
a.Primitive neuroectodermal tumor.
b.Congenital proximal sternal cleft.
c.Thoracic-type of ectopia cordis.
d.‘Cottage loaf sign’ due to right-sided diaphragmatic rupture.
Answer
c.Thoracic-type of ectopia cordis.
Discussion
Ectopia cordis (EC) is extremely rare and challenging congenital anomaly where the live, beating heart is located partially or totally outside the thoracic cavity with or without a pericardial covering. Depending upon the displacement of the heart, EC is classified into five types: cervical, cervicothoracic, thoracic, thoracoabdominal, and abdominal.1 The hypothesis regarding the etiology of EC involves abnormalities in the development of mesoderm during early embryogenesis. The failure of cephalic anterior folds to fuse in a timely manner may result in this anomaly. Complete or incomplete failure of midline fusion at this stage results in disorders varying from isolated EC to complete ventral evisceration.2 EC with a midline supraumbilical abdominal-wall defect (omphalocele), caudosternal cleft, anterior diaphragmatic hernia along with pericardial and intracardiac defects constitute pentalogy of Cantrell association.3 The most common intracardiac defect is ventricular septal defect followed by atrial septal defect, valvular or infundibular pulmonary stenosis, tetralogy of Fallot, and left ventricular diverticulum (in the order of decreasing prevalence).4 EC can be noted as early as 10–12 weeks of pregnancy using three-dimensional ultrasound with a combination of Doppler sonography.5,6 Antenatal magnetic resonance imaging (MRI) is also useful in planning management of complicated anomalies. While EC generally is sporadic, it has been linked with chromosomal abnormalities (e.g., trisomy 18, Turner syndrome, 46, XX, and 17q+).4 Early diagnosis would allow informed clinical decision making on the part of physician and family. EC has a complicated prognosis related to the severity of intrinsic cardiac defects and associated congenital anomalies. Most cases are stillborn or die within the first hours or days of life and therefore require tertiary-level intensive care from birth. It is fatal if left untreated, and the ultimate management is surgical correction.5 Labor room resuscitation includes coverage of the exposed heart and viscera with saline-soaked gauze pads wrapping to prevent desiccation and heat loss due to increased risk of hypothermia, and to prevent trauma and infections. Immediate surgical correction is often difficult due to the inability to enclose the ectopic heart within a hypoplastic thoracic cage, and also due to the abnormal course, length, and positioning of the great vessels making them vulnerable to kink and further compromised circulation.7 Hence, the treatment usually consists of staged-surgical repair in highly specialized centers and should be considered in all cases, taking comorbidities into account.
Conclusion
Prenatal ultrasound and antenatal MRI play a vital role in classification, prognostication, and management of this complex and challenging condition. Regular antenatal follow-up in a high-risk obstetric clinic and during delivery considering the possibility of safe in utero transfer to a tertiary care center is recommended. A multi-disciplinary team approach consisting of an obstetrician, radiologist, neonatologist, pediatric surgeon, and cardiothoracic surgeon should create a care plan along with the family in deciding on the viability, mode of delivery, and various postnatal management options.
references
- Alphonso N, Venugopal PS, Deshpande R, Anderson D. Complete thoracic ectopia cordis. Eur J Cardiothorac Surg 2003 Mar;23(3):426-428.
- Shad J, Budhwani K, Biswas R. Thoracic ectopia cordis. BMJ Case Rep 2012 Sep;2012.
- Cantrell JR, Haller JA, Ravitch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet 1958 Nov;107(5):602-614.
- Engum SA. Embryology, sternal clefts, ectopia cordis, and Cantrell’s pentalogy. Semin Pediatr Surg 2008 Aug;17(3):154-160.
- Repondek-Liberska M, Janiak K, Wloch A. Fetal echocardiography in ectopia cordis. Pediatr Cardiol 2000 May-Jun;21(3):249-252.
- Harrison MR, Filly RA, Stanger P, de Lorimier AA. Prenatal diagnosis and management of omphalocele and ectopia cordis. J Pediatr Surg 1982 Feb;17(1):64-66.
- Carmi R, Boughman JA. Pentalogy of Cantrell and associated midline anomalies: a possible ventral midline developmental field. Am J Med Genet 1992 Jan;42(1):90-95.