Pertussis (whooping cough) is a highly contagious disease caused by Bordetella pertussis. The most severe symptoms occur in infants and young children. The disease is milder and unnoticeable among adolescents, young adults, and adults, who constitute a reservoir and a source of infection spread to infants and young children.1 Although pertussis may occur at any age, most cases of serious disease and fatalities occur in early infancy.2–5
Pertussis continues to be a major public health concern globally. It tends to be a cyclic disease, peaking every three to five years. Despite the high global coverage reported among infants, pertussis is still considered the main cause of morbidity and fatality among infants and young children.6
As of 2016, the World Health Organization (WHO) estimated 142 512 pertussis cases and around 89 000 deaths in children aged < 5 years along with an estimated 86% diphtheria-tetanus-pertussis 3 (DTP3) coverage.6 Oman is not an exception as we observe a growing number of pertussis cases among young infants. To our knowledge, there is a dearth of published studies describing the epidemiology of pertussis in Oman. In this report, we describe the progress made towards pertussis control and the challenges ahead to achieve and maintain control in Oman.
Oman had a total population of 3 992 893 in 2014. Nearly 44% of the population is non-Omani and includes many resident workers and their families from South and Southeast Asia. Children under five years of age comprised 9.5% of the population, whereas those less than 15 years of age comprised 22%.7,8
Methods
Pertussis data were collected from 1981 to 2015 from various sources including Annual Health Reports,7 annual Ministry of Health (MOH) progress reports, ex. Directorate General of Health Affairs (DGHA), and Community Health and Diseases Surveillance Newsletter, which provided information for the calculation of different pertussis indicators. These included pertussis incidence, DTP3 coverage, case demographics (age, residency, vaccination status), and the proportion of clinical cases meeting the definition that were laboratory tested. The incidence of pertussis per 100 000 population was also calculated. Data analysis was conducted using Epi-Info 6 software with a p-value < 0.050 as a cut-off point for significance of the association using the chi-square test.
Between 1981 and 2015, a suspect case was defined as a person with a cough and at least one of the following symptoms: paroxysms of coughing, inspiratory ‘whoop’, post-tussive vomiting (i.e., vomiting immediately after coughing), white blood cell count with absolute lymphocytes over 15 000 per cubic millimeter or more, and cases that were clinically or laboratory-confirmed.9,10
Nasopharyngeal swab and culture are used to confirm B. pertussis etiology and to evaluate the contribution of other Bordetella species. Multitarget polymerase chain reaction assays were also performed on specimens from pertussis cases collected from clusters and outbreaks and submitted to the clinical microbiology laboratory at Royal Hospital or Oman’s Central Public Health Laboratory (CPHL), a WHO Accredited Regional Reference Laboratory.
A confirmed case is a suspect case with positive isolation of B. pertussis, a significant rise in specific antibody (e.g., IgG or IgA), or with an epidemiological link (e.g., household contact with laboratory-confirmed case within 28 days before or after the onset of illness in the subject).
The contacts of a confirmed case of pertussis were to be managed as follow: keep infants and young children away from the patient at hospital or at home. Those known to have been in close contact with the patient were to be given chemoprophylaxis with erythromycin 30–50 mg/kg body weight per day for 14 days along with verifying the vaccination status of those close contacts, and where appropriate, vaccinating them with diphtheria-tetanus-whole cell pertussis (DTwP) vaccine.9
All pertussis-related activities, including vaccination and laboratory testing of suspected pertussis cases, are financially supported by the government and provided free of charge at government health facilities for all nationals and non-national residents. Vaccinations with DTwP vaccine and for other diseases are provided in all primary health care centers (public institutions) and some private institutions certified for vaccination.
A unique defaulter retrieval system was also introduced to complement the program. Parents of any child who missed the vaccination appointment are called by the Extended Programme of Immunization (EPI) staff nurse. If there is no response from the parents for more than two weeks, a home visit is carried out by a public health sanitarian.11
Furthermore, by 1994, the MOH established a policy that requires a proof of completion of all routine vaccinations including DTwP3 and booster vaccines, for any eligible child and/or person upon school entry at any level. Children or any eligible person with an incomplete or unknown vaccination status are required to complete the recommended childhood schedule. Pertussis vaccination with tetanus, diphtheria and acellular pertussis (Tdap) vaccine for health care workers (HCW) was implemented in 2012.
All health facilities forward the vaccination coverage data monthly to the EPI section at the Department of Communicable Disease Control (CDC), MOH. DTwP vaccine coverage is calculated using DPT3 administered as the numerator, and the number of surviving infants or the number of infants and children registered at health facilities as the denominator. Data on DTP vaccination coverage and other statistics are analyzed at both governorate and national levels, and feedback is regularly sent to all health facilities and concerned health authorities.9-11 In addition, the administrative coverage is confirmed by periodic validation of the vaccination coverage data using data quality self-assessment every three years and coverage evaluation surveys every five years.
Pertussis notification is integrated into the national communicable diseases surveillance system. This system monitors pertussis incidence and other notifiable vaccine-preventable diseases. The MOH requires all health facilities, including private institutions, notify suspect pertussis cases within seven days along with vaccination status and contacts. Regular reporting from all institutions must be done promptly, including zero reporting of pertussis. The MOH periodically sends communications to all doctors reminding them about the requirement for timely and zero reporting of pertussis. Governorate epidemiologists are assigned at all governorates to oversee surveillance activities.11
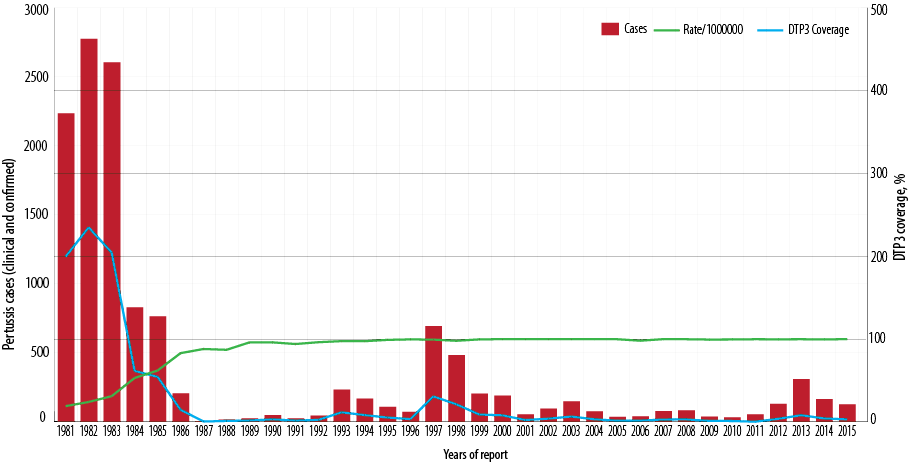
Figure 1: Number of reported pertussis cases and the incidence rate of pertussis and diphtheria-tetanus-pertussis vaccine 3 (DTP3) coverage (%) by year of report, Oman, 1981–2015.
As per the national policy, an outbreak is defined as the occurrence of a cluster of five or more suspected cases, or a confirmed case of pertussis11 and warrants immediate initiation of outbreak control activities. According to the national guidelines, all outbreaks should be investigated within 48 hours. Clinical specimens should be obtained from all suspected cases and sent to the CPHL within three days. In addition, contacts of suspected pertussis cases including infants, children of ≥ 1 year, and adults are checked for their vaccination history. If there is no evidence of vaccination, vaccination is warranted. All outbreaks of pertussis are investigated with particular reference to age, sex, vaccination status, and history of contact with pertussis cases.
Results
In 1981, vaccination with DTwP vaccine was introduced into EPI for children at three, five, and seven months of age. In response to a pertussis outbreak in 1997, by January 1998, DTwP vaccination was rescheduled to six weeks, three months, five months, and a booster at 15 months of age. In January 2008, the DTwP was rescheduled to be given at two, four, and six months, and a booster at 18 months. In 2003, pentavalent vaccine (DTwP, Haeomophilus Influenzae type b [Hib] and Hepatitis B [HepB]) was introduced at the same age (two, four, and six months). In January 2015, pentavalent vaccine at two and four months was replaced with hexavalent (DTaP, Hib, HepB, and inactivated polio vaccine [IPV]). The proportion of young children who were vaccinated with DTwP3 rose from approximately 19% in 1981 to 97% in 1992 and has since been maintained at that level [Figure 1]. DTwP3 and DTwP boosters (18 months) have been maintained at ≥ 97% nationally and provincially (governorate) since, as well as timeliness of vaccination at ≥ 97%. The administrative coverage has been confirmed by coverage evaluation surveys conducted in 2008.12 Nationally, Tdap vaccination coverage of healthcare workers has been reported at between 60–70%.
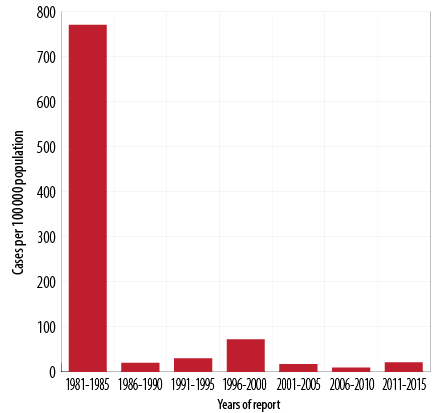
Figure 2: Pertussis incidence rates by year of report, Oman, 1981–2015.
Since the introduction of DTP vaccine in 1981, the overall incidence of pertussis dropped dramatically by late eighties. The incidence rate per 100 000 population has fallen nationwide from an average of 771 cases per 100 000 population between 1981 and 1985 to 20 cases per 100 000 population between 1986 and 1990, 72 cases per 100 000 population from 1996 to 2000, nine cases per 100 000 population between 2006 and 2010, and 21 cases per 100 000 population between 2011 and 2015. Between 1981 and 2015, the pertussis incidence rate fell from 771 to 21 per 100 000 population (p < 0.001) [Figure 2].
Between 2011 and 2015, a total of 831 cases were notified, of which 785 (94.4%) met the pertussis case definition, and 119 (15.1%) yielded positive for pertussis. Due to the difficulties associated with the laboratory confirmation of pertussis, only outbreaks or clusters were investigated.Out of the total cases, 203 (25.8%), 183 (23.3%), 157 (20.0%), 105 (13.3%), and 83 (10.5%) were reported from North Sharqiyah, Muscat, North Batinah, Dhofar, and South Batinah governorates, respectively [Figure 3].
In the vaccine era and since 1987, the pertussis cases in Oman are cyclical, with peaks every three to five years [Figure 1]. Subsequently, three major outbreaks of pertussis were reported in some parts of the country. In 1997, a total of 694 laboratory-confirmed cases were reported from North Batinah governorate with an incidence rate of 31 per 100 000 population (694/2 256 000*100 000). The age distribution of the cases revealed that 490 (70.6%) occurred in infants ≤ three months of age giving an incidence rate of 4 702 cases per 100 000 population under three-month-olds (490/10 420*100 000). Nearly all cases were hospitalized. These cases were unvaccinated as they were not of age for the first dose of DTwP1 vaccine. In response to pertussis outbreak, in January 1998, DTwP vaccination was rescheduled to be given at six weeks, three and five months, and a booster at 18 months. The second outbreak was reported in 2003, with 149 pertussis cases notified [Figure 1] giving an incidence rate of 6 per 100 000 population (149/2 340 851*100 000).
The last large pertussis outbreak (30 cases) was reported in 2013 with a rate of 8 per 100 000 population (250/3 855 206*100 000). Out of the total, 168 cases were notified among infants < 2 months old with the rate of 1 390 per 100 000 population (168/12 081*100 000). Of those, 112 (36.2%) and 62 (20.0%) cases were reported from North Sharqiyah and Muscat governorates, respectively.
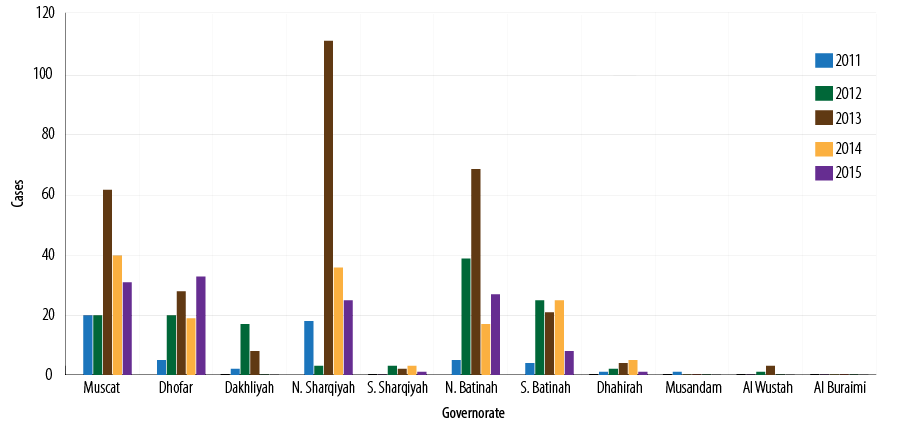
Figure 3: Number of reported pertussis cases by governorates, Oman, 2011–2015.
Between 2011 and 2015, the overall total number of cases reported was 785 (average 157). Of the total, 625 (79.6%) infants were less than 12 months old (average disease rate 185 per 100 000 population), and almost all patients were hospitalized. The incidence rates per 100 000 population showed a downward trend (373, 195, and 136 in 2013, 2014, and 2015, respectively) [Figure 4]. No deaths recorded were attributed to pertussis based on mortality since 2007.7
Between 2011 and 2015, the total recorded cases for infants aged < 2 months was 357/625 (57.1%) [Figure 5], (average disease incidence is 600/100 000 population), for 2–3 months was 129/625 (20.6%) (average disease incidence is 202/100 000 population), for 4–6 months was 80/625 (12.8%) (average disease incidence is 119/100 000 population), and for 6–12 months was 59 (9.4%) (average disease incidence is 22/100 000 population). The majority of cases (486/625; 77.8%) were in infants aged < 4 months. The trend of pertussis disease incidence/100 000 population among infants < 2 months old was 295, 520, 1390, 446, and 353 in 2011, 2012, 2013, 2014, and 2015, respectively. In 2013, the highest pertussis rates observed were among all age groups younger than 12 months [Figure 6].
Of the total, 160/785 (20.3%) cases were in infants ≥ 12 months old (rate 4/100 000 population). Downward trend rates were reported among the 5–14 year age groups, and a very low disease rates were observed among in the > 15 years of age [Figure 6].
Surveillance indicators have been monitored nationally and provincially since the introduction of case-based surveillance in 1996. The timeliness and completeness of reporting and investigation of suspect cases have been ≥ 95%. Outbreaks or cluster cases investigated have had clinical specimens collected, transported to, and tested by the national laboratory promptly.
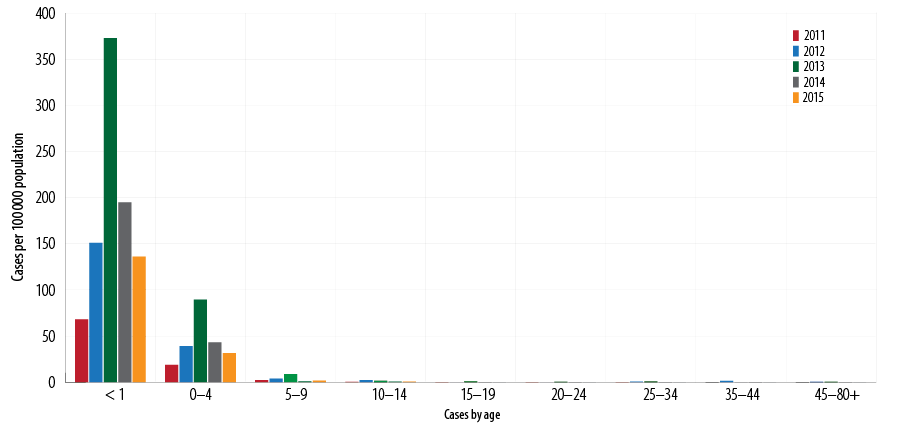
Figure 4: Pertussis incidence rates per 100 000 population by age, Oman, 2011–2015.
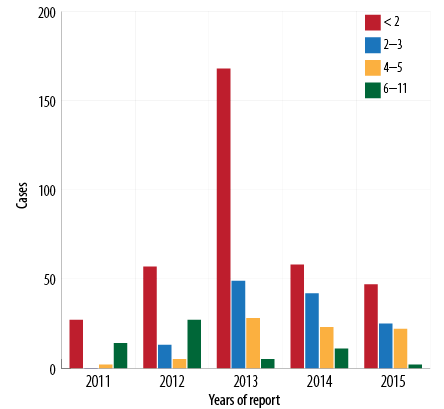
Figure 5: Pertussis cases by age group (< 12 months) and year of report, Oman, 2011–2015.
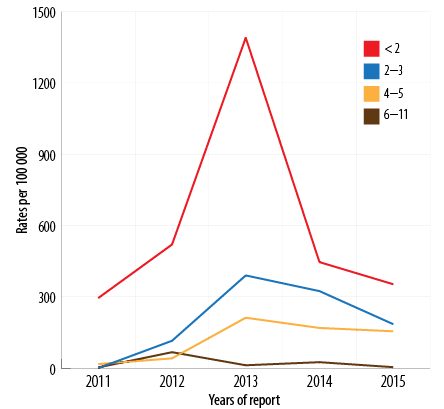
Figure 6: Pertussis incidence by age group (< 12 months) by year of report, Oman, 2011–2015.
Discussion
Oman has made progress towards pertussis control goals by implementing two main strategies: achieving and sustaining high vaccination coverage and population immunity and developing an efficient surveillance system.
The first strategy of sustaining high vaccination coverage has been achieved through high routine coverage (DTP3), reaching ≥ 97% (recommended ≥ 90%7) nationally and provincially. The second strategy, developing case-based surveillance for pertussis was implemented. Over the last decade, Oman has achieved all the targeted pertussis surveillance performance indicators.
The introduction of an infant vaccination program in 1981 was associated with a steep decline in the number of pertussis cases. Similar findings were reported from the US.12,13
Despite maintaining a high DTP3 and booster doses coverage rate (≥ 97%) among preschool-aged children, treatment of pertussis cases to prevent further spread of disease, and use of antimicrobial prophylaxis in contacts of pertussis cases, the control of pertussis has been a continuous challenge in Oman, which has experienced an increased periodic outbreaks. The outbreak and incidence show seasonal variations within years and epidemic cycles over multiple years with peaks occurring at three to five-year intervals with major outbreaks occurring in 1997 and 2013 as the number of susceptible persons in the population increased. Similar epidemiological situations have been observed in the countries of the European Union/European Economic Area (EU/EEA), with a growing number of pertussis cases, mostly in very young infants, adolescents, and adults.14
In the vaccine era, almost 79.6% of pertussis cases were observed in children aged < 12 months (rate of 185 per 100 000 population) and 77.8% were < 4 months. Notably, the incidence was highest in infants < 2 months old followed by 2–4 month olds, which indicated that these infants were not protected.6,13,15 Infants aged < 12 months have the greatest risk of hospitalization for complications and death from pertussis.13 Similar findings were observed in a local study, where 95% of the pertussis cases were in infants and 75.5% were aged < 4 months.16 Studies from the US reported the pertussis burden remains the highest in infants, who also have the highest rates of complications and death. In the US and England, 91% of pertussis cases among < 12 month olds were hospitalized.17–20
Studies have demonstrated efficient transplacental transfer of anti-pertussis antibodies to the fetus, which protects the infant until they are old enough to receive the first dose of DTP vaccine at two months.6 Adolescents and adults serve as reservoirs and sources of transmission of pertussis to very young infants who are unimmunized or partially immunized and thus more vulnerable to disease-related complications and higher mortality.2–4 Therefore, the focus of prevention and control efforts should be a priority for the protection of infants from the severe form of disease. Vaccination of pregnant women using a single dose of Tdap could be provided during a prenatal care visit between 20 and 22 weeks of pregnancy to maximize the maternal antibody response and passive antibody transfer to the infant. This is likely to be the most effective strategy for preventing disease in infants too young to be vaccinated against DTP.13,18 A study from the UK indicated a high impact on infant pertussis-related morbidity and mortality with Tdap vaccination of pregnant women.21 In addition, Oman widely implemented cocooning strategy by vaccinating adolescents (booster) and health care professionals who had not previously received Tdap, which may have an impact on disease prevention in our settings with high DTP3 coverage.6
Cases in adolescent and adults have also been detected and no significant disease incidence was observed. Similar findings were observed in Argentina15,19 and Canada.22–24 This could be attributed to low awareness among HCW of pertussis in the adolescent and adult population, resulting in under-diagnosed and/or underreported pertussis cases. Most of these infections are subclinical or mild, and prolonged cough may be the only clinical feature in adolescents or adults, who may present for diagnosis late or not at all.25 Other factors include the tendency of HCW to report only severe cases, and lack of widespread use of enzyme-linked immunosorbent assay test, which is the preferred lab-method for adults.
Our study has some limitations including information on DTP history among cases that were not systematically collected; therefore, the rates of cases among vaccinated and unvaccinated were not calculated. Another limitation is that the majority of sporadic cases were diagnosed as clinical pertussis therefore, some of these cases might be due to B. parapertussis.
Adolescents and adults are known to be significant sources of transmission of B. pertussis to unvaccinated young infants. A systematic review of the source of infection in infants aged < 6 months demonstrated that household contacts were the source of B. pertussis in 74%–96% of cases.26 Therefore, vaccination of adolescents using a single dose of Tdap in place of Td provided at 18 years would minimize the source of infection among household contacts.6
Conclusion
Oman has shown a high DTP3 coverage over the years; however, pertussis remains a challenge to control in infants under 12 months old. Therefore, Tdap vaccination of pregnant women would help decrease the burden of pertussis since the majority of cases are children < 2 months of age who are too young to be vaccinated. In addition, there is an urgent need to improve hospital-based surveillance, particularly laboratory-confirmed disease and assessment of disease burden, with a particular focus on morbidity and fatalities in infants < 12 months old.
Disclosure
The authors declared no conflict of interest. No funding was received for this study.
Acknowledgements
We are in debt to H.E. Dr. Ali Bin Moosa, late Dr. Ali Jaffer Mohammed, Late H.E. Dr. Ahmed Al Ghassani,
Late Dr. Musallam Al Buali, H.E. Dr Mohammed Al Hossani, Mr. Islam Al Bulushi, Mrs. Maryam Al Shueibi, Mr. Hasamudin Mohammed Nwar Alden, Mr. Baddar Al Rawahi, Mr. Salim Al Mahrouqi, Dr. Suleiman Al Busaidi, Dr. Said Al Baqlani,
Dr. Hanan Al Kindi, Mr. Arumugam Raju, Dr. Shyam Bawikar, Dr. Idris Al Obaidani, Mrs. Halema Al Bulushi,
Dr. Mohammed Farag, Dr. Jeffrey V. Singh, all Governorate EPI focal points and epidemiologists, Dr. Aleksandra Caric, Miss. Adithya sharanya, and Miss. Magda Salim Al Wahebi, for their vision and support.
references
- 1. World Health Organization. Immunization, Vaccines and Biologicals. Pertussis [cited 2017 September]. Available from: www.who.int/immunization/diseases/pertussis/en/.
- 2. Cherry JD. Epidemiology of pertussis. Pediatr Infect Dis J 2006 Apr;25(4):361-362.
- 3. Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, et al; Infant Pertussis Study Group. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J 2007 Apr;26(4):293-299.
- 4. Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, et al. Infant pertussis: who was the source? Pediatr Infect Dis J 2004 Nov;23(11):985-989.
- 5. Lavine J, Broutin H, Harvill ET, Bjørnstad ON. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine 2010 Dec;29(1):11-16.
- 6. World Health Organization. The weekly epidemiological record (WER) 2017;90(44):661–680 [cited 2017 September]. Available from: http://www.who.int/wer/en.
- 7. Ministry of Health. Oman. Annual Health Report 2015. Directorate General of Planning. Department of Information and Statistics; 2015.
- 8. Health Facts 2014, Ministry of Health, 2014 [cited: 2017 September]. Available from: https://www.moh.gov.om/documents/274609/274943/%D8%AD%D9%82%D8%A7%D8%A6%D9%82+%D8%B5%D8%AD%D9%8A%D8%A9+2014/cad173e0-bebf-45bd-a49a-80ced22fbfc8.
- 9. Ministry of Health. Manual on expanded program on immunization. Department of surveillance and disease control 2002. Directorate General of Heath Affairs [cited 2017 September]. Available from: http://www.cdscoman.org/uploads/cdscoman/EPI_Manual.pdf.
- 10. Al Awaidy ST. Impact of strategies and activities for reducing morbidity and mortality of vaccine-preventable diseases in Oman: A status report. J Vaccines Immun 2015;3(1):1-6.
- 11. Ministry of Health. Communicable disease surveillance & control 2012. Expanded Programme on Immunization (EPI) [cited 2017 September]. Available from: http://www.cdscoman.org/immunization.html.
- 12. The Oman World Health Survey, 2008 [cited 2014 October]. Available from: https://drive.google.com/file/d/0B07Z7alv9G2gWWJfeVM2LTFrcXc/view.
- 13. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011 Oct;60(41):1424-1426.
- 14. European Centre for Disease Prevention and Control. Expert consultation on pertussis, Barcelona, 20 November 2012. Stockholm: ECDC; 2014.
- 15. Hozbor D, Mooi F, Flores D, Weltman G, Bottero D, Fossati S, et al. Pertussis epidemiology in Argentina: trends over 2004-2007. J Infect 2009 Oct;59(4):225-231.
- 16. Al Maani A, Al Qayoudhi A, Nazir HF, Omar H, Al Jardani A, Al Muharrami Z, et al. Pertussis and Pertussis like Illness: Pediatric Experience in Oman. Oman Med J 2017 Sep;32(5):396-402.
- 17. Pertussis - United States 1997-2000 [cited 2017 September]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5104a1.htm.
- 18. Pertussis [cited 2017 September]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pert.pdf.
- 19. Nardone A, Pebody RG, Maple PA, Andrews N, Gay NJ, Miller E. Sero-epidemiology of Bordetella pertussis in England and Wales. Vaccine 2004 Mar;22(9-10):1314-1319.
- 20. Public Health England 1998-1995. Enhanced Pertussis Surveillance 2016 [cited 2017 September]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/521442/Figure_3_Incidence_of_laboratory-confirmed_pertussis__by_total_case-patients_and_age_group_in_England__1998_-_2015_.pdf.
- 21. Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014 Oct;384(9953):1521-1528.
- 22. Halperin SA. Pertussis immunization for adolescents: What are we waiting for? Can J Infect Dis 2001 Mar;12(2):74-76.
- 23. De Serres G, Shadmani R, Duval B, Boulianne N, Déry P, Douville Fradet M, et al. Morbidity of pertussis in adolescents and adults. J Infect Dis 2000 Jul;182(1):174-179.
- 24. Senzilet LD, Halperin SA, Spika JS, Alagaratnam M, Morris A, Smith B; Sentinel Health Unit Surveillance System Pertussis Working Group. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin Infect Dis 2001 Jun;32(12):1691-1697.
- 25. Pertussis vaccines: WHO position paper. Weekly epidemiological record August 2015, 90, 433-460 [cited 2017 September]. Available from: http://www.who.int/wer/2015/wer9035.pdf.
- 26. Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine 2013 Jan;31(4):618-625.