Endometriosis is a common gynecological problem in women of reproductive age. It frequently occurs in the pelvis. Extrapelvic endometriosis, though rare, has been reported in the urinary tract, gastrointestinal tract, thorax, and abdominal wall.1 Abdominal wall endometriosis (AWE) mostly occurs following gynecological surgery.2 It is also referred to as scar endometriosis (SE) or incisional endometriosis (IE).2 It is often misdiagnosed due to its nonspecific symptoms like vague abdominal pain and swelling.3 Accurate and early diagnosis of endometriosis obviates unnecessary diagnostic procedures. It has well-defined cytological features. Fine-needle aspiration cytology (FNAC) plays a significant role in the timely diagnosis of SE.4,5 This case report presents one such rare case where FNAC played a vital role in patient management.
Case report
A 35-year-old woman presented with an abdominal lump that she noticed three years ago. The lump gradually increased in size. It was associated with intermittent pain and not related to her menstrual cycle. She had undergone an uncomplicated cesarean section eight years before. She had no other significant medical history. Ultrasonography showed a well-defined hypoechoic lesion in the anterior abdominal wall in the subcutaneous plane. The clinical diagnosis was desmoid tumor. She was sent to the cytopathology section for FNAC. Examination revealed a well-healed cesarean section scar. The swelling was in the right iliac fossa 2–3 cm away from the scar and measured about 3 × 3 cm in size. It was well-defined, firm, non-mobile, non-tender, and located above the plane of rectus sheath. FNAC yielded a fatty material, which was spread onto clean glass slides. The smears were stained with hematoxylin and eosin (H&E) and Leishman stain. Microscopy revealed two cell populations: epithelial cells, which were polygonal with round to oval nuclei with uniform bland chromatin and a moderate amount of cytoplasm arranged in flat sheets and honeycomb pattern, and spindle-shaped cells in clusters [Figure 1a-d]. The background showed many pigment-laden macrophages with cyst macrophages and red blood cells [Figure 1d inset].
A cytological diagnosis of endometriosis was made. The patient underwent wide local excision of the mass. The histopathology section received a fatty specimen measuring 3 × 3 × 2 cm, with the cut surface showing cystic areas along with grey-white and hemorrhagic areas.
H&E stained sections showed fibrocollagenous tissue intermixed with cystically dilated endometrial glands and endometrial stroma surrounded by hemorrhagic and edematous areas [Figure 2a]. The histopathological examination confirmed the diagnosis of endometriosis [Figure 2b]. The patient continues to be followed-up.
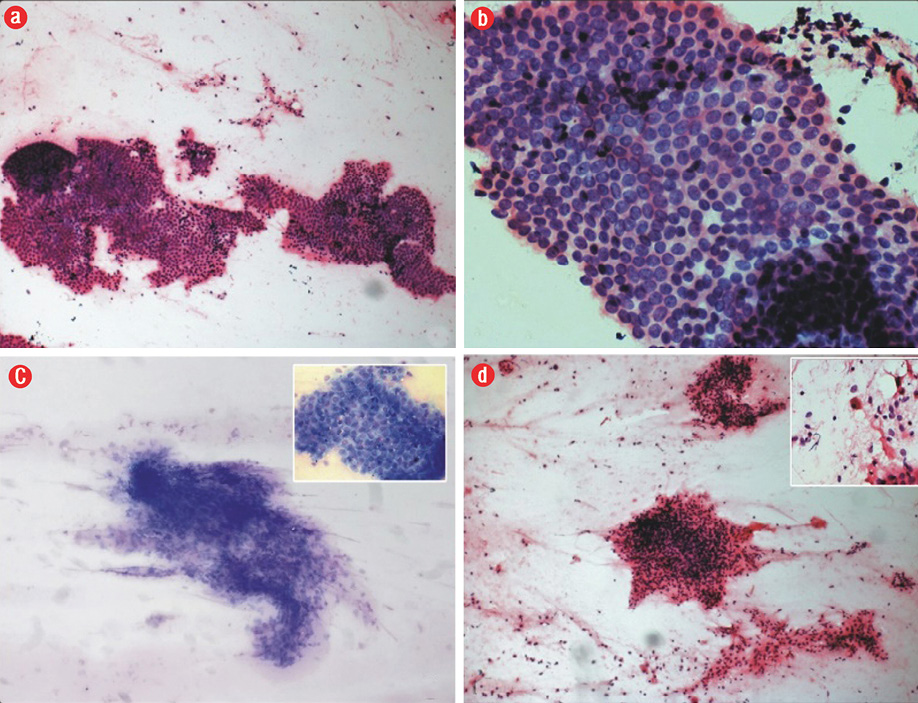
Figure 1: (a) Photomicrography of the epithelial cells arranged in a honeycomb pattern (hematoxylin and eosin staining (H&E)), magnification = 100 ×, (b) honeycomb pattern of epithelial cells (H&E), magnification = 400 ×, (c) spindle-shaped stromal cells in cluster (Leishman stain), magnification = 100 ×; inset, honeycomb pattern of epithelial cells (Leishman), magnification = 400 ×, and (d) stromal cell cluster (H&E), magnification = 100 ×; inset, hemosiderin-laden macrophage in the background (H&E), magnification = 400 ×.
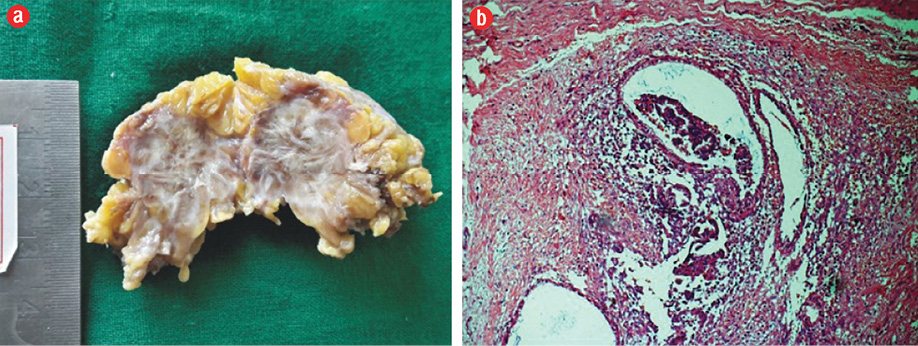
Figure 2: (a) Gross picture of the specimen, cut surface showing grey-white with hemorrhagic and tiny cystic areas. (b) Photomicrography of histopathological section showing endometrial glands and stroma in the fibrocollagenous background (hematoxylin and eosin staining), magnification = 100 ×.
Discussion
AWE is defined as endometrial tissue superficial to the peritoneum.6 It appears around a previously healed surgical scar, which frequently follows gynecological surgeries. SE has been reported in the literature since 1956.7 Its occurrence following cesarean section is uncommon with an incidence of 0.03% to 0.4%.1,7 It has often been underreported and clinically mistaken for a hernia, suture granuloma, desmoid tumor, nodular fasciitis, lipoma, sarcoma, or metastatic malignancy. It is a diagnostic dilemma to the clinicians as it lacks conclusive results using imaging techniques.3 None of the available biochemical markers are diagnostic of endometriosis.8 However, SE usually presents as a subcutaneous firm mass and has definitive cytomorphological features. FNAC being a simple and non-invasive technique is the investigation of choice in cases of SE for accurate and timely preoperative diagnosis.3 FNAC can also be used as a tool to exclude other differentials in cases of abdominal wall mass.4
Endometriosis is common in women of reproductive age, rarely occurring in postmenopausal women. It is an estrogen-dependent lesion. Its rare occurrence in postmenopausal women has been related to hormone replacement therapy.9 SE may develop after gynecological surgeries like cesarean section, hysterectomy, hysterotomy, and rarely after fallopian tube surgeries, amniocentesis, and episiotomy. Its occurrence after hernia repair, laparotomy, and at laparoscopic trocar site has also been reported.1,2
Endometriosis can develop in the abdominal wall without a prior surgery. In such cases, it is often restricted to the skin, most commonly at the umbilicus and rarely at the vulva, perineum, groin, and extremities.2,6 In a study by Ecker et al,2 out of 65 patients with AWE, six had no history of prior surgery. Of the six, four presented with lumps in the umbilicus and two as groin masses.
Presenting symptoms of SE are vague in most cases. A typical symptom is a lump in or around the surgical scar with an increase in lump size, skin discoloration and pain during menstruation.8 These symptoms are often absent, and the patient is referred to the surgeon for investigation into hernia and other surgical conditions.3 In our case, patient presented with a swelling in the right iliac fossa associated with vague pain and the clinical diagnosis of desmoid tumor. The lump of SE can develop from three months to 10 years after the surgery.10 Our patient presented with a lump five years after cesarean section. Imaging studies do not contribute significantly towards the diagnosis. However, they are used to assess the extent and nature of the lesion.6
FNAC of endometriosis has a specific cytological picture. It demonstrates sheets of epithelial cells, spindled stromal cells, and a variable number of hemosiderin-laden macrophages. Presence of any of the two features is diagnostic of endometriosis.7 The reporting cytopathologist must be aware of this for a timely and accurate diagnosis. The cytological features of endometriosis can vary according to cyclic hormonal changes. In the proliferative phase, glandular cells are seen as honeycomb sheets and in tight clusters and appear bland. Spindle stromal cells are in the form of syncytial clusters. In the secretory phase, glandular cells show an increase in cell size and microvacuolation. Stromal cells reveal abundant cytoplasm. Cells in IE can also undergo a wide spectrum of morphological changes, which can result in an inaccurate diagnosis of malignant neoplasm. Epithelial cells may show squamous, mucinous, or tubal metaplasia. Nuclear atypia may be seen during the secretory phase. Stromal elements can develop decidual or myxoid change.4,7,8,10 Malignant transformation, though rare, is a documented complication of IE.5 The malignancies reported include endometrioid carcinoma, clear cell carcinoma, sarcoma, mucinous, and serous carcinoma.5,9 The reporting pathologist must also be aware of these variations and report appropriately with advice on biopsy for definitive diagnosis when a typical picture is not evident.5,7
The lesions in the differential diagnosis of AWE have well-defined cytological features. Desmoid tumor and fibrosis show less cellularity with benign-appearing spindle-shaped cells. Suture granuloma shows nonspecific inflammation with or without granulomatous reaction with a foreign body. Lipoma shows mature adipose tissue and fibrous tissue fragments. Nodular fasciitis shows myxoid background and pleomorphic cells. Smears from primary or metastatic malignancies show hypercellularity and frankly atypical cells.4 Clinically, a hernia demonstrates a cough impulse and can be differentiated by radiological investigations.6
According to the metastatic theory transfer of endometrial cells to adjacent location via surgical manipulations, hematogenous, or lymphatic dissemination result in endometriosis.
SE is explained by the same theory.6 Wide surgical excision with at least a 1 cm margin and patch grafting of the defect is the treatment of choice for SE, and it seldom reoccurs.6
Conclusion
SE is a diagnostically challenging entity. It must be considered in the differential diagnosis of any anterior abdominal wall swelling in a woman of reproductive age. FNAC must be the first modality of investigation as it is simple, non-invasive, and cost-effective, and can accurately and quickly diagnose a case of SE. At times, atypical features may be encountered and biopsy is advised to rule out the possibility of malignancy.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Machairiotis N, Stylianaki A, Dryllis G, Zarogoulidis P, Kouroutou P, Tsiamis N, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013 Dec;8(1):194.
- 2. Ecker AM, Donnellan NM, Shepherd JP, Lee TT. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol 2014 Oct;211(4):363.e1-363.e5.
- 3. Blanco RG, Parithivel VS, Shah AK, Gumbs MA, Schein M, Gerst PH. Abdominal wall endometriomas. Am J Surg 2003 Jun;185(6):596-598.
- 4. Pachori G, Sharma R, Sunaria RK, Bayla T. Scar endometriosis: Diagnosis by fine needle aspiration. J Cytol 2015 Jan-Mar;32(1):65-67.
- 5. Rekhi B, Sugoor P, Patil A, Shylasree TS, Kerkar R, Maheshwari A. Cytopathological features of scar endometriosis mimicking an adenocarcinoma: A diagnostic pitfall. J Cytol 2013 Oct;30(4):280-283.
- 6. Cihangir U, Ahmet G, Hasene Ö, Kemal D. Scar endometriosis: A case report of this uncommon entity and review of the literature. Case Rep Obstet Gynecol 2013;2013,article ID 386783, 4 pages.
- 7. Veda P, Srinivasaiah M. Incisional endometriosis: diagnosed by fine needle aspiration cytology. J Lab Physicians 2010 Jul;2(2):117-120.
- 8. Poflee S, Bode A, Mahana S. Cytodiagnosis of scar endometriosis. Cytojournal 2014 Jan;11:1.
- 9. Bhat RA, Teo M, Bhat AK. Endometriosis after surgical menopause mimicking pelvic malignancy: surgeons’ predicament. Oman Med J 2014 May;29(3):226-231.
- 10. Pathan ZA, Dinesh U, Rao R. Scar endometriosis. J Cytol 2010 Jul;27(3):106-108.