Spontaneous coronary artery dissection (SCAD) is commonly overlooked in patients presenting with acute coronary syndrome (ACS). It was first described in 1931 following the autopsy of a 42-year-old woman who died suddenly.1
The incidence varies from 0.1%−0.28% in patients with ACS or sudden cardiac death.2,3 With the advent of coronary intravascular ultrasound (IVUS) and optical coherence tomography (OCT), a higher incidence of 4% has been reported.4 SCAD remains a poorly understood condition with no specific management guidelines.
Case report
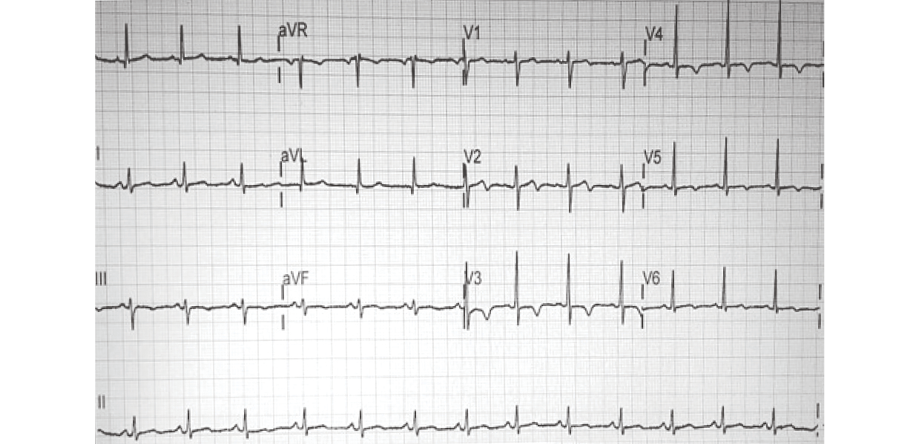
Figure 1: On presentation, the patient’s 12-lead electrocardiogram showed normal sinus rhythm with new T-wave inversions in leads V3-V6 and a biphasic T-wave in lead V2.
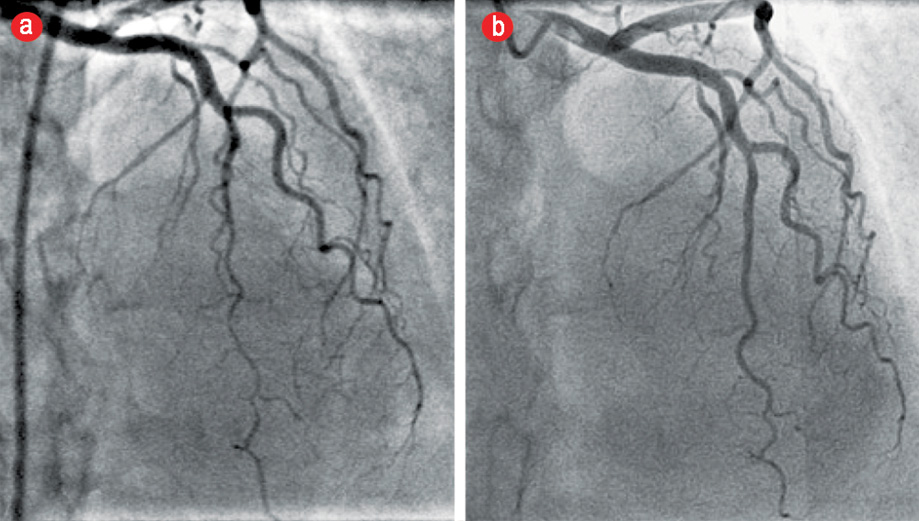
Figure 2: Angiogram of the left anterior descending (LAD) coronary artery in the cranial projection showing a type 2 Spontaneous coronary artery dissection. (a) Acutely tapered segment in the distal LAD with reverse tapering towards the terminal end. (b) The same projection on an angiogram three months later showed complete resolution of the dissection with conservative management.
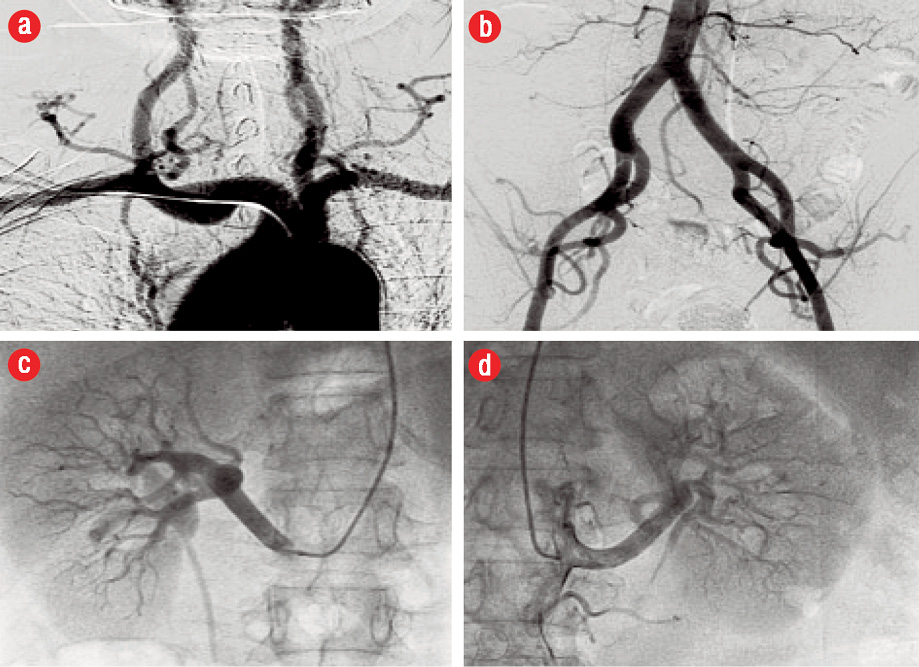
Figure 3: Angiographic surveillance for fibromuscular dysplasia (FMD). (a) Extracerebral head and neck arterial circulation, (b) distal aortic and iliofemoral arterial bed, (c) right renal, and (d) left renal arteries. The vessels were angiographically normal with no evidence of FMD.
A 55-year-old diabetic and hypertensive woman presented to a tertiary care hospital with typical ischemic chest pain lasting for 30 minutes.
On presentation, she was hemodynamically stable with no abnormal clinical findings. Electrocardiography showed dynamic T-wave inversions in the anterolateral precordial leads [Figure 1]. Her initial troponin T level was elevated and peaked at 134 pg/mL (normal range 0−14 pg/mL). She was treated as non-ST-elevation myocardial infarction (NSTEMI). She underwent coronary angiography, which revealed no angiographic evidence of coronary atherosclerosis. The distal segments of the major epicardial vessels followed an extraordinarily tortuous course. There was an acutely tapered segment in the distal left anterior descending (LAD) with reverse tapering towards the terminal end [Figure 2]. The appearance was consistent with type 2 SCAD.5
The patient subsequently presented to the cardiology clinic at another institution for ongoing follow-up. She remained asymptomatic after discharge. An echocardiogram done at this time showed mild segmental left ventricular (LV) systolic dysfunction with mild anterior wall hypokinesis. There were no significant valvular lesions noted. She was advised to undergo a repeat coronary angiography to study the progression of LAD disease. An elective repeat coronary angiogram three months after the index event revealed angiographically smooth coronary arteries. The previously diseased distal LAD segment had completely healed, and the vessel had remodeled to its normal morphology [Figure 2]. Selective renal, aorta-iliac, and extracerebral carotid angiography showed no vascular abnormalities. In particular, there was no angiographic evidence of fibromuscular dysplasia (FMD) in the respective arterial beds [Figure 3].
Discussion
Our patient had an ACS secondary to a non-atherosclerotic, non-FMD-related SCAD with complete resolution of the dissection with conservative medical management as documented on the control cineangiography three months after the index event.
SCAD is defined as the non-traumatic and non-iatrogenic separation of the coronary arterial wall by intramural hemorrhage creating a false lumen, with or without an intimal tear. It is believed to be multifactorial. Young females of childbearing age appear to be particularly at risk in their early peripartum period. Other risk factors include exercise, systemic inflammatory conditions, connective tissue disorders, and autoimmune disorders.2,6
The angiographic diagnosis is challenging. It requires the presence of a non-iatrogenic dissection in the absence of coronary atherosclerosis appearing as a radiolucent intimal flap or typical contrast staining at the site of dissection. These stereotypical changes are seen in < 30% of cases. The more common finding is long, diffuse, narrowing due to intramural hematoma.7,8
Saw proposed a simple classification system for SCAD based on angiographic analysis.5 Type 1 describes the pathognomonic multiple radiolucent lumen with contrast wall staining. In the most common variant, type 2 SCAD, a long diffuse (typically > 20 mm) smooth stenosis is noted with abrupt change in the caliber of the involved segment. There is smooth tapering followed by reverse tapering more distally. Type 3 describes focal or tubular stenosis that mimics atherosclerotic plaque. Intracoronary imaging is needed in such cases to confirm SCAD.5,9 Advanced imaging modalities like IVUS and OCT are the gold standard for
diagnosing SCAD.9,10
SCAD also poses major therapeutic challenges given limited evidence to guide management. Therefore, the choice of medical treatment, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) is largely dictated by the clinical presentation and the degree of compromise to coronary flow on angiographic study. A retrospective analysis of 189 patients with SCAD showed that 90% of patients managed conservatively had an uneventful hospital course with no increase in early mortality. PCI outcomes were disappointing, even in patients with preserved coronary flow. This was related to the frailty of the diseased vessel and the propagation of dissection with further instrumentation. At five-year follow-up, the rates of recurrent SCAD were similar in the revascularized and non-revascularized groups.11 Another group from Vancouver prospectively followed 168 patients with SCAD and reported similarly discouraging PCI outcomes with a successful outcome in less than two-thirds of patients treated. Conservative management was associated with spontaneous healing. In this series, recurrence was documented in 13.1% of the patients at two-year follow-up.12 Therefore, the consensus favors adopting a conservative strategy in stable patients. This includes treatment with aspirin and beta-blockers to modify shear forces across delicate arterial segments. The role of antiplatelet agents and anticoagulants in SCAD is not well studied. Based on expert opinion, dual antiplatelet therapy can be administered for one year, followed by lifelong aspirin for secondary prevention.6,13 Anticoagulation, if initially started, should be discontinued once SCAD is diagnosed due to a potential risk of extension of the dissection.13
In young women without conventional atherogenic risk factors presenting with ACS due to SCAD, FMD must be excluded. This nonatherosclerotic, noninflammatory hyperproliferative condition affects arteries causing stenosis, occlusion, dissection, and aneurysms. The condition can affect any arterial bed including coronary arteries, but renal and carotid arteries are most frequently involved.14 Saw et al,12 reported that up to 86% of patients diagnosed with SCAD had underlying coronary FMD. To ascertain the diagnosis, FMD should be visualized in other arterial territories. Coronary FMD can manifest in various angiographic forms. The most common is SCAD but also include distal tapering or smooth narrowing, unusual tortuosity, and spasm.8
Data on natural history emanate from case series with varied management strategies. Therefore, prognosis remains unclear. However, from the largest series by the Vancouver group, the rate of recurrent in-hospital myocardial infarction was 4.5%, and long-term major adverse coronary event rate occurred in 20% of patients.12 The Mayo Clinic group reported a 17% recurrence rate of SCAD over a 10-year period.15 Some investigators suggested that exaggerated coronary tortuosity was associated with increased risk of recurrent SCAD.16
Conclusion
SCAD should be suspected in young patients presenting with ACS, especially in the absence of traditional atherogenic risk factors. The coronary cineangiogram should be carefully scrutinized for the subtle angiographic features characterizing the condition. Detecting SCAD has major bearings on acute management of such patients by deferring unwarranted and potentially hazardous pharmacological therapy and unnecessary invasive interventions. Care should be taken to tailor therapy to the case at hand given the lack of evidence to guide treatment with an emphasis on conservative management in stable patients.
Disclosures
The authors declared no conflicts of interest.
references
- 1. Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ 1931: Ruptupe/ Apri1:667.
- 2. Sultan A, Kreutz RP. Variations in Clinical Presentation, Risk Factors, Treatment, and Prognosis of Spontaneous Coronary Artery Dissection. J Invasive Cardiol 2015 Aug;27(8):363-369.
- 3. Hoye A. Spontaneous coronary artery dissection: time for a concerted effort to better understand this rare condition. J Invasive Cardiol 2010 May;22(5):229-230.
- 4. Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2013. Epub ahead of print.
- 5. Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014 Dec;84(7):1115-1122.
- 6. Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013 Sep;29(9):1027-1033.
- 7. Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013 Jan;6(1):44-52.
- 8. Michelis KC, Olin JW, Kadian-Dodov D, d’Escamard V, Kovacic JC. Coronary Artery Manifestations of Fibromuscular Dysplasia. Journal of t h e American college of Cardiology, 2014: 64(10); 1033-1046.
- 9. Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv 2016 Feb;87(2):E54-E61.
- 10. Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv 2012 Oct;5(10):1062-1070.
- 11. Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014 Dec;7(6):777-786.
- 12. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014 Oct;7(5):645-655.
- 13. Saw J, Mancini GB, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2016 Jul;68(3):297-312.
- 14. Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012 Jun;125(25):3182-3190.
- 15. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012 Jul;126(5):579-588.
- 16. Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014 Oct;7(5):656-662.