Case report
A 55-year-old Omani man, with no history of major illnesses or hospitalizations was admitted to our hospital with high fever. The illness started two weeks prior to his admission with high fever, rigors, and loose motion, which settled spontaneously after three days. The man worked on an oilrig in Laos, Cambodia. Five days prior to his return to Oman, he had accidental trauma of his right shoulder during work. He developed severe shoulder pain and high-grade fever with chills and rigors. When he arrived in Oman, he sought a consultation from a private hospital where magnetic resonance imaging (MRI) of his shoulder showed a ligamental tear and mild joint effusion with no evidence of septic arthritis. At the same time, he was noted to have high fever with elevated random blood sugar and raised inflammatory markers. The patient was transferred to our hospital for further evaluation and management.
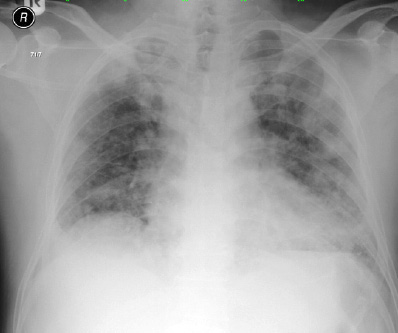
Figure 1: Chest X-ray on admission was unremarkable.
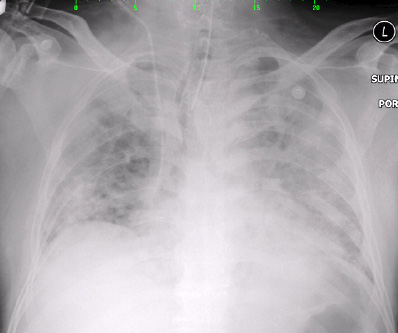
Figure 2: Chest X-ray on day two of admission showed right upper zone haziness with bilateral pulmonary congestion.
On arrival to our hospital, he appeared sick, febrile (38.5 oC), and tachycardiac but conscious and alert. Initial chest, heart, and abdominal examination were unremarkable. Similarly, his initial complete blood count (CBC) showed normal hemoglobin levels and white cell counts. However, his inflammatory marker levels, including C-reactive protein and erythrocyte sedimentation rate, were abnormally high (400 mg/L and 103 mm/hr, respectively). Malaria screening was negative. The patient started empirical treatment with piperacillin-tazobactam 4.5 g three times daily. However, he deteriorated rapidly, developed sudden shortness of breath, severe cough, hypoxia, and hypotension requiring oxygen and inotropic support.
Right upper zone haziness with bilateral pulmonary congestion were seen on chest radiography [Figure 1 and Figure 2] for which intravenous clarithromycin 500 mg twice daily was prescribed. Serum for Legionella pneumophila, Mycoplasma pneumoniae, and Coxiella burnetti serology was collected. Bedside echocardiography was performed and showed good systolic function with no evidence of vegetation.
Blood cultures flagged positive with gram-negative bacilli that grew as dull, wrinkled colonies with corrugated surface, umbonated centers and radiating ridges on the bacterial culture media. They appeared as non-lactose fermenters in the MacConkey agar with positive oxidase reaction. Gram’s stain revealed bipolar staining (safety pin appearance). Further testing showed growth at 42 o C, nitrate reduction, and arginine decarboxylase positivity [Table 1]. Final identification was made using the API 20 enteric (API 20E) and API 20 nonenteric (API 20NE) (bioMerieux/ Marcy 1'Etoile, France) biochemical tests, whichidentified the growth as Burkholderia pseudomallei.
The antibiotic susceptibility testing by disk diffusion and automated microbroth dilution testing (Phoenix; BD Diagnostics Maryland, US) revealed resistance to multiple antibiotics including aminoglycosides (gentamycin and amikacin), ampicillin, and ciprofloxacin and sensitivity to amoxicillin-clavulanic acid, ceftazidim, piperacillin-tazobactam, carbapenems, and co-trimoxazole, and intermediate susceptibility to levofloxacin.
Table 1: Phenotypic characteristics of the strain.
|
Oxidase |
+ |
|
Motility |
+ |
|
Growth on MacConkey agar |
+ |
|
Growth at 42 ºC |
+ |
|
Nitrate reduction |
+ |
|
Indole |
- |
|
Citrate |
- |
|
Arginine dihydrolase |
+ |
|
Urea |
- |
|
Gelatin |
- |
When the positive blood culture was determined, the patient was already intubated, ventilated, and transferred to the intensive care unit (ICU) with multiorgan failure including acute renal failure and acute respiratory distress. Initial growth identification and preliminary report of the suspected organism was communicated with the caring physicians. His antibiotics were modified accordingly.
The patient continued to be critically ill and highly febrile requiring high ventilator settings. Subsequent blood cultures continued to grow the same organism for two weeks despite the use of appropriate broad-spectrum antibiotics and meropenem. Chest computed tomography (CT) showed bilateral consolidation with cavitation. With worsening of patient condition, meropenem was later changed to ceftazidime, and colistin and tigecycline were then added to cover for the nosocomial acquisition of multidrug resistant Acinetobacter baumanii in the endotracheal tube (ETT) secretions and worsening pulmonary infiltrates on chest radiography.
His condition continued to deteriorate despite maximal antibiotic coverage and ICU support. He progressed to persistent hypotension and asystole and died after three weeks of ICU admission.
Discussion
Melioidosis, also known as Whitmore’s disease, is an infection caused by the gram-negative bacteria B. pseudomallei. This is an endemic pathogen in Southeast Asia and Northern Australia, where it is commonly associated with community-acquired infections.1,2 This organism is an aerobic, non-spore forming, motile, gram negative bacillus with a “safety-pin appearance” when seen under light microscope. It can grow in any routine culture media.
It naturally inhabits soil and water such as rice paddies, stagnant water and moist soils,3 which are believed to be the primary sources for acquiring infection by susceptible hosts.
Thailand has the largest number of cases reported annually, with an incidence rate of 3.6–5.5 per 100 000 population4 accounting for 39% of community-acquired septicemia deaths5 with 36% of those dying from community-acquired pneumonia.6 Occupational exposure to contaminated soil and water has been reported frequently and together with diabetes mellitus are considered the most common risk factors associated with the development of melioidosis.7 Other reported risk factors include renal disorders, chronic liver disease, alcoholism, steroid therapy, cancer or other immune-suppressing disorders, and thalassemia.3
Our patient was diagnosed with diabetes mellitus just prior to admission to our hospital. Diabetes has been found to be the single most common predisposing factor for septicemic melioidosis.7
Melioidosis is called the “great imitator” because it can present with a wide spectrum of clinical presentations, including severe disease with high fatality. The disease can become latent for months or years before its clinical manifestations.
The incubation period of melioidosis is wide and varies significantly between individuals ranging from two days to 26 years.8
Clinically, infections may appear in its acute form, spreading fulminant sepsis, or subclinically, lasting for a long period. The most common manifestations are bacteraemia, pneumonia, localized pulmonary, splenic, hepatic, or cutaneous abscesses and, rarely, osteomyelitis and lymphadenitis.9
B. pseudomallei can readily grow in any standard culture media such as blood, MacConkey, or cystine-lactose-electrolyte deficient agars, and routine blood culture broth. Selective media (e.g., Ashdown’s agar) is required for respiratory tract specimens to selectively isolate the organism from the normal respiratory flora.10 It produces dry wrinkled colonies. It is considered a category 3 pathogen for which all laboratory work should be carried out inside a biosafety cabinet.11 It must be emphasized that the diagnosis of melioidosis requires a high index of suspicion. Although the organism is easy to isolate and identify, it may be confused as an environmental contaminant such as pseudomonas species. A number of immunological and molecular approaches including enzyme-linked immunosorbent assay (ELISA),12 latex agglutination and polymerase chain reaction (PCR) primers have been developed with reasonable sensitivity
and specificity.13
B. pseudomallei is intrinsically resistant to many commonly used antibiotics such as penicillin, ampicillin, first and second generation cephalosporins, macrolides, rifampicin, and aminoglycosides.14 Ceftazidime or imipenem are considered the treatment of choice for severe melioidosis.15 Both of these antibiotics were given to the patient at regular doses.
The mortality rate of this infection remains high in cases of acute septicemic melioidosis with multiorgan failure, especially if blood culture continues to be positive after the first week of hospitalization with use of appropriate antibiotics.16 Therefore, a prolonged follow-up, with repeated blood cultures, appears to be essential in the management of melioidosis.17
Oral maintenance combination therapy using chloramphenicol, co-trimoxazole, and doxycycline appears to reduce the risk of relapse to less than 5%, but this regimen is associated with a greater number of adverse effects. Patients who present with mild or localized disease may be treated with oral agents alone. The total duration of antibiotic treatment should not be less than 12–20 weeks with life-long follow-up.16
Conclusion
Due to its severity, melioidosis should be considered as a differential diagnosis in individuals working or returning from endemic regions and presenting with pneumonia or sepsis. This should prompt clinicians to take a travel history to any of the endemic areas. In addition, correct identification of B. pseudomallei is necessary for effective treatment and has to be intensive and prolonged. Therefore, a high index of suspicion should also be kept at laboratory level.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010 Nov;4(11):e900.
- 2. Currie BJ1. Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg 2008 Dec;(102)(Suppl 1):S1-S4.
- 3. Dance DA. Melioidosis as an emerging global problem. Acta Trop 2000 Feb;74(2-3):115-119.
- 4. Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, Smith MD, et al. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol 1994a Oct;23(5):1082-1090.
- 5. Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, Davis TM, et al. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis 1989 May;159(5):890-899.
- 6. Boonsawat W, Boonma P, Tangdajahiran T, Paupermpoonsiri S, Wongpratoom W, Romphryk A. Community-acquired pneumonia in adults at Srinagarind Hospital. J Med Assoc Thai 1990 Jun;73(6):345-352.
- 7. Currie BJ, Fisher DA, Howard DM, Burrow JN, Selvanayagam S, Snelling PL, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop 2000 Feb;74(2-3):121-127.
- 8. Leelarasamee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis 1989 May-Jun;11(3):413-425.
- 9. Brett PJ, Woods DE. Pathogenesis of and immunity to melioidosis. Acta Trop 2000 Feb;74(2-3):201-210.
- 10. Sirisinha S, Anuntagool N, Dharakul T, Ekpo P, Wongratanacheewin S, Naigowit P, et al. Recent developments in laboratory diagnosis of melioidosis. Acta Trop 2000 Feb;74(2-3):235-245.
- 11. Casey Ch L. Deborah E. W. Biosafety in Microbiological and Biomedical Laboratories, 5th Edition, 2009 December [cited 2016 Nov 7]. Available from: http://www.cdc.gov/biosafety/publications/bmbl5/index.htm.
- 12. Vadivelu J, Puthucheary SD, Gendeh GS, Parasakthi N. Serodiagnosis of melioidosis in Malaysia. Singapore Med J 1995 Jun;36(3):299-302.
- 13. Wongratanacheewin S, Komutrin K, Sermswan RW. Use of multiplex PCR patterns as genetic markers for Burkholderia pseudomallei. Acta Trop 2000 Feb;74(2-3):193-199.
- 14. Ashdown LR. In-vitro activities of the newer ß-lactam and quinolone agents against Pseudomonas pseudomallei. Antimicrob Agents Chemother 1988;32:1435-1436.
- 15. Chaowagul W. Recent advances in the treatment of severe melioidosis. Acta Trop 2000 Feb;74(2-3):133-137.
- 16. Currie BJ, Fisher DA, Howard DM, Burrow JN. Neurological melioidosis. Acta Trop 2000 Feb;74(2-3):145-151.
- 17. Limmathurotsakul D, Wuthiekanun V, Wongsuvan G, Pangmee S, Amornchai P, Teparrakkul P, et al. Repeat blood culture positive for B. pseudomallei indicates an increased risk of death from melioidosis. Am J Trop Med Hyg 2011 Jun;84(6):858-861.