Vaginal atrophy is a common condition found in up to 45% of women, especially menopausal women.1–3 Clinicians diagnose this condition on the basis of various symptoms and standard local examination findings.4 Vaginal pH and the vaginal maturation index (VMI) can be used to support the diagnosis if needed.1 Untreated symptoms, in addition to causing discomfort, can have adverse effects on women’s sexual life and overall well-being.5,6 Various treatment options are available for treating the symptoms related to vaginal atrophy. Non-hormonal products like lubricants, moisturizers, jellies, douches, and herbal medications have been used by women suffering from these complaints for many years.
The use of exogenous estrogen (oral, vaginal, or transdermal) improves blood flow to vaginal mucosa, increases local secretions, and thickens vaginal epithelium in patients with vaginal atrophy. Several studies conducted in western countries have shown that using a local vaginal estrogen cream is beneficial over systemic estrogen treatment for patients with vaginal atrophy.7 Local estrogen replenishes healthy microflora of the vagina and improves the pH of vaginal tissue.4
Our study sought to determine the effects of using a vaginal estrogen cream in the treatment of vaginal atrophy in menopausal women in India.
Methods
This prospective interventional study took place from June 2012 to November 2013 and was approved by the Scientific Ethics and Research Committee (SERC) of the Government Medical College. Sixty menopausal women attending the gynecology outpatient department of a tertiary care hospital of Gujarat, India, were screened for eligibility for inclusion into the study. Of these, 50 women between 40 to 80 years old with a symptom score of at least five (with at least one symptom of vaginal atrophy (vaginal dryness, itching, burning, and dyspareunia) rated as moderate or severe and a Genital Health Clinical Evaluation (GHCE) score8 of less than 15 were included in the study. Each woman gave their written informed consent.
Women having any cervical pathology (determined by speculum examination or Pap smear), an endometrial thickness > 5 mm and/or enlarged ovaries seen on transvaginal ultrasound, bleeding from the uterus, using any form of estrogens or natural estrogen products eight weeks prior to the start of the study, or a history of any thromboembolic episode, venous thromboembolism, liver diseases, breast cancer, or other medical problems were excluded from the study.
All the patients applied intravaginal conjugated equine estrogen (CEE) vaginal cream 0.5 g (equivalent to 0.3 mg) twice a week for 12 weeks. The medication was applied with a gentle measure applicator. The patients were educated regarding drug application, and the first application was supervised.
The patients were followed at four, eight, and 12 weeks of treatment. At the final visit, patients were asked to bring the empty bottle of medication to ensure that they completed the full course of treatment, thus ensuring compliance. At every visit, patients underwent a physical and pelvic examination. During follow-up visits, symptomatic evaluation was done. The efficacy at the end of 12 weeks was assessed by comparing symptom score, GHCE score, vaginal pH, and VMI to baseline scores.
The symptom score was evaluated on a four-point scale (0 - no complaint, 1 - mild, 2 - moderate, 3 - severe). A baseline composite score was calculated for four symptoms (vaginal dryness, itching, burning, and dyspareunia). The GHCE score considered six parameters (vaginal pH, fluid secretion, moisture, vaginal rugosity, mucosal color, and epithelial mucosa) and was measured on a scale of one to four.
During each follow-up visit, the safety of medication was assessed by measuring combined endometrial thickness with transvaginal ultrasound in all women. Combined endometrial thickness > 5 mm was considered a sign of endometrial hyperplasia, and a criterion to withhold treatment. Any side effects were noted and treated according to the standard guidelines.
Descriptive statistics (mean, standard deviation (SD), and range) were recorded for the baseline parameters (age and years since menopause). The difference in vaginal symptoms and VMI score after 12 weeks compared to baseline was assessed using the Wilcoxon Signed Ranks test. The variance test was used to evaluate the change in GHCE score.
Results
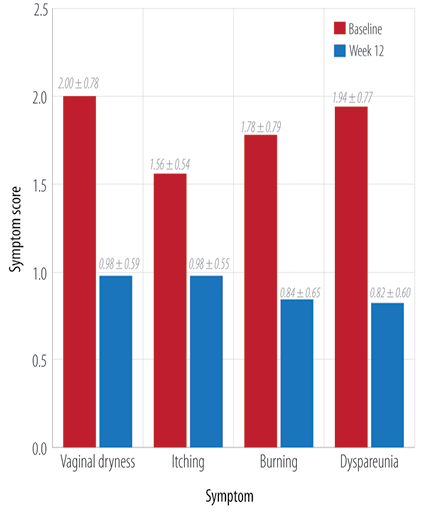
Figure 1: Mean individual symptom score at baseline and end of the study.
The mean age of patients was 54.3 years. The mean duration of time since starting menopause was 9.2 years (range = 2–25 years). Many of these patients also had other menopausal symptoms. Sixteen patients complained of hot flushes and night sweats, and 10 patients had urinary problems (increased frequency or incontinence). Five patients also had sleep disturbances while three had increased anxiety or irritability.
Table 1: Most bothersome symptom of vaginal atrophy reported by menopausal women (n = 50).
|
Vaginal dryness |
19 (38.0) |
|
Itching |
3 (6.0) |
|
Burning |
12 (24.0) |
Table 2: Change in Genital Health Clinical Evaluation (GHCE) score over the study period.
|
Baseline |
7.8+2.5 |
|
4 |
12.3+2.5 |
|
8 |
14.7+2.6 |
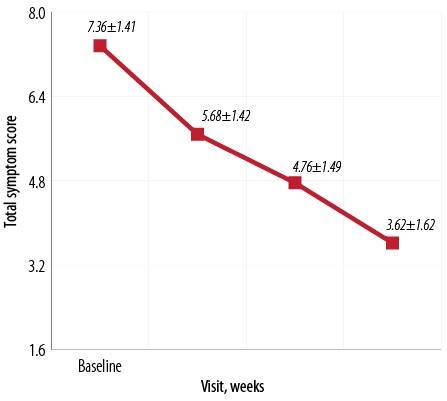
Figure 2: Mean total symptom score at baseline and weeks four, eight, and 12 of the study.
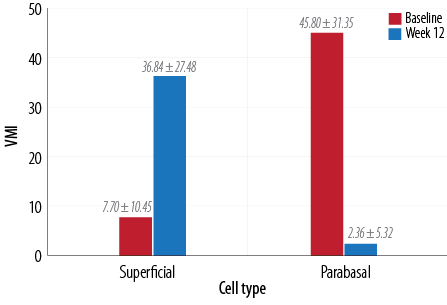
Figure 4: Change in mean vaginal maturation index (VMI) score in superficial and parabasal cells at baseline and week 12.
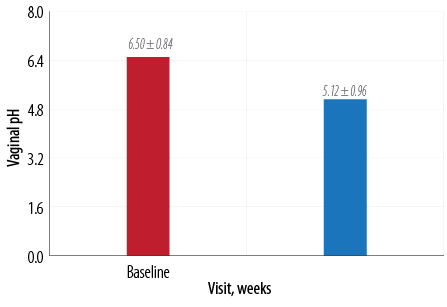
Figure 3: Change in mean vaginal pH at baseline and week 12.
Table 1 shows the most bothersome symptoms reported by patients. At baseline, vaginal dryness was reported as the most bothersome symptom followed by dyspareunia and vaginal burning. Itching was least commonly reported as the most bothersome symptom.
After 12 weeks, all symptoms showed statistically significant improvement (p < 0.010) at the individual level [Figure 1]. A significant improvement (p < 0.010) in the mean total symptom score for all 50 patients was also seen at the end of the study [Figure 2]. The study medication was well tolerated with all 50 patients completing the treatment during study period without any adverse events.
Similarly, a significant (p < 0.010) improvement was also noted in vaginal pH [Figure 3]. VMI score for both superficial and parabasal cells was statistically significant (p < 0.010; Figure 4).
Analysis of variance for the GHCE scores showed a significant visit wise improvement (p < 0.010; Table 2).
Discussion
Vaginal dryness and dyspareunia followed by burning in the genital area were the most bothersome symptoms in many postmenopausal women. The North American Menopause Society (NAMS) position statement in 2013 stated that many vaginal atrophy cases remain under diagnosed, but can be successfully treated with estrogen therapy once diagnosed.8 One study (REVIVE) used a survey to assess women’s knowledge of vulvar and vaginal atrophy record attitudes about interactions with healthcare providers. The symptoms of vaginal atrophy among postmenopausal women reported were vaginal dryness (55%), dyspareunia (44%), vaginal irritation (37%), vaginal tenderness (17%), bleeding during intercourse (8%), and pain during exercise (2%).9
It may take two or more weeks to prime the vaginal epithelium after starting local estrogen therapy and see noticeable results.4 In our study, a significant improvement was seen in all symptoms (total symptom score) at the end of four weeks and the end of the 12-week therapy. This study demonstrated the efficacy of vaginal CEE cream in patients aged 40–80 years with 2–25 years since the start of menopause.
After menopause, because of the reduced levels of estrogen levels, epithelial cells and their glycogen content is less. The rise in vaginal pH in postmenopausal patients is due to the availability of less glucose for conversion to lactic acid.4 A pH > 5 is considered consistent with vaginal atrophy.10 In this study, we observed a significant reduction in pH from 6.5 to 5.1 after 12 weeks of treatment with local CEE. Possibly, with a longer duration, pH might be further improved. Long-term studies are required to confirm this finding. Typically in routine clinical practice, pH testing is not done. However, these findings are important because the consistency of improvement in pH is associated with overall and individual symptom improvement. In other studies, local vaginal estrogen therapy has been found to be effective in decreasing vaginal discomfort and improve dyspareunia.10,11
Quantitative measure (i.e., VMI) is used for diagnosis in clinical trial settings. VMI shows the relative proportions of parabasal, intermediate, and superficial vaginal epithelial cells. A lower number of superficial cells indicates low estrogen levels in the local tissue.4 We measured VMI at baseline and after 12 weeks of treatment and found a significant improvement in both superficial and parabasal cells of the vagina.
Vaginal atrophy, because of its chronic nature, may require long-term treatment. Local estrogen therapy has been recommended as long as patients have symptom-related discomfort.12 According to recently published global consensus statement on menopausal hormonal therapy, local low-dose estrogen therapy is preferred in women whose symptoms are limited to vaginal dryness or discomfort during intercourse.13
Many women having symptoms of vaginal atrophy do not discuss them with their healthcare provider. The REVIVE study findings showed that about 40% of women expected their health care provider to initiate a conversation about menopausal symptoms.9 Hence, it is important to talk to patients about the problems associated with vaginal atrophy since treatment with topical estrogen is effective in improving symptoms. If women do not report these symptoms, they may go untreated.14,15 As the current treatment guidelines for vaginal atrophy recommend the use of the lowest effective dose of local estrogen therapy for symptom relief,5 we used CEE vaginal cream 0.5 g (equivalent to 0.3 mg) twice a week for only 12 weeks.
Our study has a few limitations. Because of a lack of recommendations in India regarding the diagnosis of vaginal atrophy, guidelines for the use of symptom and GHCE scores were adapted from studies done in other populations. This was a single-arm study without any control group. Having a control group using standard drug therapy in the form of oral estrogen would give more information about the comparative difference in efficacy between two groups. Having an open-label study design and short therapy time are other limitations of this study.
Conclusion
Vaginal estrogen cream causes symptomatic relief in women of menopausal age in India suffering from vaginal atrophy. All fifty patients completed the treatment and reported no side effects. A multicenter trial including larger sample size representing different populations is recommended.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010 Jan;85(1):87-94.
- 2. Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med 2009 Aug;6(8):2133-2142.
- 3. Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas 2010 Nov;67(3):233-238.
- 4. Reiter S. Barriers to effective treatment of vaginal atrophy with local estrogen therapy. Int J Gen Med 2013;6:153-158.
- 5. The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013 Sep;20(9):888-902, quiz 903-904.
- 6. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999 Feb;281(6):537-544.
- 7. Bachmann G, Bouchard C, Hoppe D, Ranganath R, Altomare C, Vieweg A, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009 Jul-Aug;16(4):719-727.
- 8. North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause 2012 Mar;19(3):257-271.
- 9. Wysocki S, Kingsberg S, Krychman M. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clin Med Insights Reprod Health 2014 Jun;8:23-30.
- 10. Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol 2008 Nov;112(5):1053-1060.
- 11. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006 Oct;4(4):CD001500.
- 12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007 May-Jun;14(3 Pt 1):355-369, quiz 370-371.
- 13. de Villiers TJ, Gass ML, Haines CJ, Hall JE, Lobo RA, Pierroz DD, et al. Global consensus statement on menopausal hormone therapy. Climacteric 2013 Apr;16(2):203-204.
- 14. Moreira ED, Glasser DB, Nicolosi A, Duarte FG, Gingell C; GSSAB Investigators’ Group. Sexual problems and help-seeking behaviour in adults in the United Kingdom and continental Europe. BJU Int 2008 Apr;101(8):1005-1011.
- 15. Simon JA, Komi J. Postmenopausal women’s attitudes: vulvovaginal atrophy and its symptoms (abstract LB-10). Menopause 2007;14:1107.