Inflammatory reactions are complex responses of many tissues to various types of harmful stimuli, including irritants, foreign bodies, trauma, or pathogens. Although such type of response is beneficial, it may progress to an inflammatory disease, including rheumatoid arthritis (RA).1,2 In clinical practice, many pharmacological approaches are currently in clinical use for the treatment of RA, including disease-modifying drugs like methotrexate (MTX) and biological agents. However, the wide range of adverse effects and high cost limit their successful use, especially in low-income populations.3,4 During the inflammatory response, an exaggerated oxidative stress condition can initiate and amplify inflammatory reactions and, consequently, predispose additional tissue damage.5 Therefore, attenuation of oxidative stress with plant-derived antioxidants like polyphenols may add further therapeutic value to the currently used anti-inflammatory agents in RA.6 Moreover, many types of natural polyphenols, including silibinin, have the ability to potentially inhibit various inflammatory cascades, probably through interference with tumor necrosis factor (TNF)-α activated pathways, which are clearly defined in many in vitro and in vivo studies.7,8
Silibinin, the major active constituent of silymarin, was approved in clinical practice as a potent hepatoprotective agent9 and investigated as adjunctive treatment in cancer chemotherapy.10 Many studies proved silibinin has significant antiarthritic activity in various animal models of arthritic inflammation,11,12 and knee osteoarthritis in humans.13 However, its clinical benefit in RA is not yet evaluated in randomized clinical trials. Accordingly, our pilot clinical study was designed to evaluate the clinical benefit of silibinin, when used as an adjuvant with MTX, in patients with active RA.
Methods
We conducted a multi-center, double-blind, randomized placebo-controlled pilot clinical study with a 16-week treatment period over two years (from January 2012 to January 2014) at the Rheumatology Unit, Baghdad Teaching Hospital, Baghdad, and Al-Sader Teaching Hospital, Najaf. Of the 145 patients screened for eligibility, 78 patients with active RA using MTX (15 mg/week) were randomly selected and evaluated to participate. Only 30 patients completed the trial [Figure 1].
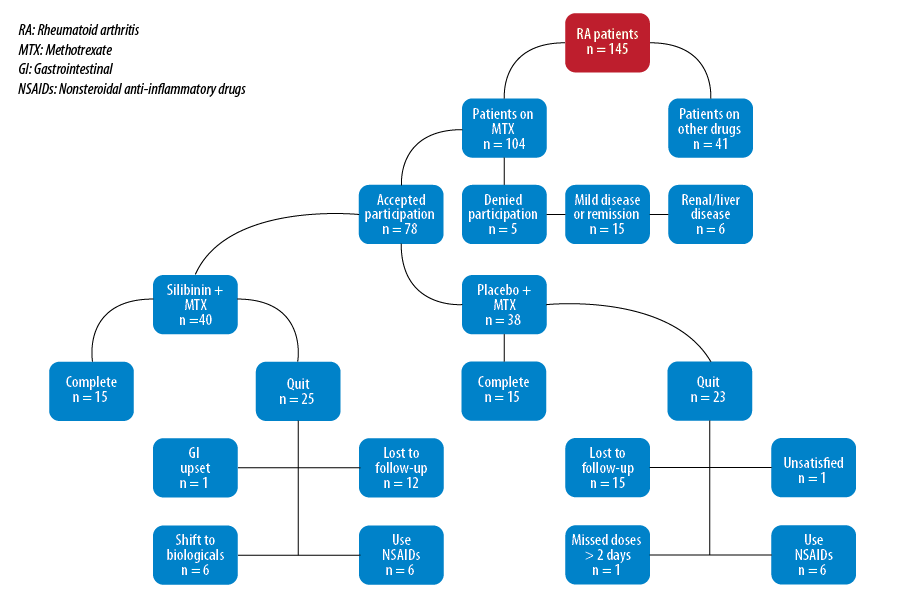
Figure 1: Flowchart of patient allocation and study follow-up.
The patients were randomly allocated to receive either silibinin (120 mg; Tolbiac S.R.L, Argentina) specially prepared as capsule dosage twice daily after a meal, or a capsule formula filled with starch as a placebo (twice daily after a meal). The two formulations were administered as an adjuvant with their regularly used MTX regimen. The patients were instructed to continue their regular drug treatment schedule and were clinically observed every four weeks for any unusual side effects.
All participants signed an informed consent form according to the principles of the Declaration of Helsinki. The local scientific ethics committee of Baghdad University, College of Pharmacy and Baghdad Teaching Hospital, and the Rheumatology Medical Department approved the study protocol. All patients included had active RA, as defined by the American College of Rheumatology (ACR) 1987 revised criteria.14 Active RA was proven by calculating either 28-joint Disease Activity Score (DAS28) or the Simple Disease Activity Index (SDAI).
All selected patients were on MTX treatment for at least three consecutive months without a clinical response until the time of inclusion. Patients with the following conditions at screening were excluded from the study: patients using non-steroidal anti-inflammatory drugs (NSAIDs) two days before inclusion; hypersensitivity or severe adverse effects to silibinin; renal or hepatic impairment; pregnant and breastfeeding women; juvenile RA; patients using disease-modifying antirheumatic drugs other than MTX or high dose steroids; missing medication for two consecutive days; coexistence of other connective tissue diseases; and mild or inactive RA.
The patient’s outcome was evaluated using the DAS28,15 SDAI,16 and functional ability by Health Assessment Questionnaire–Disability Index (HAQDI)17 at the start (baseline) and end of the 16-week study period. Moreover, evaluation of patients’ response by both ACR (ACR20, ACR50, and ACR70: ACR 20%, 50%, and 70% improvement criteria) and European League Against Rheumatism (EULAR) response criteria was done at the end of the study to compare the effect of silibinin against placebo.18 Blood samples were obtained from each patient by vein puncture at baseline and the end of the study. Of the blood collected, 3 ml was kept in an EDTA tube to be used for measurement of erythrocyte sedimentation rate (ESR) and hemoglobin (Hb).19,20 The remaining blood was transferred to a plain test tube and left at room temperature for at least 30 minutes to coagulate, and then centrifuged for 10 minutes at 4000 rpm to obtain serum. Using ready-made enzyme-linked immunosorbent assay (ELISA) kits, the resultant serum was utilized for the measurement of high-sensitivity C-reactive protein (hs-CRP), TNF-α, anti-cyclic citrullinated peptide (CCP) (DEMEDITEC, Germany), interleukin (IL)-6, IL-8, IL-2, and IL-10 (Beckman Coulter, USA).
All data were statistically analyzed using SPSS Statistics (SPSS Statistics, Chicago, US) software version 18 and Graph Pad Prism 5.1 software (Graph Pad Software Inc, California, US). Continuous variables were presented as mean ± standard error of the mean (SEM) and discrete variables presented as numbers and frequencies. The Chi-square test was used for independence to test the significance of the association between discrete variables. The paired t-test was used to evaluate the difference between pre- and post-treatment values. Moreover, one-way analysis of variance (ANOVA) was used to test the significance of the difference in means of independent samples, and supported by Bonferroni’s post hoc analysis. A p-value less than 0.050 was considered significantly different.
Results
The demographic data and baseline characteristics were not significantly different between the two groups [Table 1]. All the clinical markers evaluated were significantly improved in both groups at the end of the study compared to baseline values, except the ESR, which was not significantly altered [Table 2]. The coadministration of silibinin with MTX produces a significantly greater effect on these parameters compared to placebo except CRP and SDAI, where the post-treatment values were not significantly different (p > 0.050).
Table 1: Demographic data and baseline characteristics of the patients.
|
Age, years |
43.4±0.9 |
44.4±1.3 |
0.921 |
|
Weight, kg |
77.9±3.3 |
77.9±1.4 |
1.000 |
|
Female:Male (female) |
14:1 (93.3%) |
15:0 (100.0%) |
0.313 |
|
Disease duration, years, mean±SEM |
7.5±0.11 |
6.9±0.12 |
0.586 |
|
MTX dose mg/week, ±SEM |
12.0±0.2 |
12.0±0.2 |
1.000 |
|
Family Hx of RA |
3 (20.0%) |
2 (13.3%) |
0.621 |
|
Family Hx of CVD |
5 (33.3%) |
3 (20.0%) |
0.412 |
|
Hypertension |
6 (40.%) |
5 (33.3%) |
0.707 |
|
DM |
4 (26.7%) |
3 (20.0%) |
0.670 |
|
Positive RF |
13 (86.7%) |
12 (80.0%) |
0.624 |
MTX: methotrexate; DM: diabetes mellitus; Sc: subcutaneous;
RA: rheumatoid arthritis; RF: rheumatoid factor; CVD: cardiovascular disease; Hx= history; SEM: standard error of the mean.
Table 2: Effect of silibinin on ESR and CRP levels and the clinical evaluation scores of patients with active RA maintained on MTX.
|
TJC |
14.1±0.23 |
9.6±0.37*a |
14.7±0.23 |
13.1±0.22*b |
|
SJC |
6.2±0.2 |
4.9±0.18*a |
5.7±0.18 |
5.1±0.2*b |
|
VAS, mm |
70.5±0.89 |
58.0±1.5*a |
68.8±1.0 |
62.5±1.0*b |
|
EGA, cm |
7.1±0.2 |
4.9±0.24*a |
6.1±0.2 |
5.5±0.16b |
|
ESR, mm/hr |
59.8± 3.0 |
48.1±2.0*a |
61.5±2.1 |
57.6±2.3b |
|
CRP, mg/dL |
1.0±0.01 |
0.94±0.001*a |
1.01±0.01 |
0.98±0.007*a |
|
DAS28 |
6.9±0.06 |
5.8±0.07*a |
6.6±0.1 |
6.1±0.1*b |
|
SDAI |
35.9±0.3 |
26.6±0.27*a |
35.1±0.3 |
26.4±0.21*a |
*significantly different compared to pre-treatment (p < 0.050); post-treatment values with different superscripts (a,b) within each parameter are significantly different (p < 0.050). RA: rheumatoid arthritis; TJC: tender joint count; SJC: swollen joint count; VAS: visual analogue scale; EGA: evaluator global assessment; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; DAS28: 28-joint disease activity score; SDAI: simple disease activity index; HAQDI: health assessment questionnaire disability index.
Over half (60.0%) of the patients in silibinin group achieved ACR20 compared to 40.0% of patients in the placebo group, but the difference was not statistically significant [Table 3]. Additionally, 26.7% of patients taking silibinin with MTX achieved ACR50 compared to 6.6% in the placebo group (p = 0.142). Moreover, only one patient (6.6%) in silibinin group achieved ACR70 while no patients in the placebo group achieved ACR70 (p = 0.309). Regarding EULAR response, 53.3% of patients using silibinin and 33.3% of patients receiving placebo achieved EULAR moderate response, but the difference was not statistically significant (p = 0.269). Two patients (13.3%) in the silibinin group had a good EULAR response compared to no patients in the placebo group (p = 0.143). Similar results were obtained in EULAR non-responders. We observed a high level (> 1) in all functional areas of HAQDI score for RA patients in both silibinin and placebo groups [Table 4]. The values of all functional areas were significantly decreased in both groups compared to baseline values. However, silibinin, when coadministered with MTX, produces a significantly (p < 0.050) greater effect in dress, arise, and activity scores compared to that reported in the placebo group.
Table 3: Effect of silibinin on ACR and EULAR response criteria of patients with active RA maintained on methotrexate.
|
ACR20 |
9 (60.0%) |
6 (40.0%) |
0.268 |
|
ACR50 |
4 (26.7%) |
1 (6.6%) |
0.141 |
|
ACR70 |
1 (6.6%) |
0 (0.0%) |
0.312 |
|
EULAR good response |
2 (13.3%) |
0 (0.0%) |
0.138 |
|
EULAR moderate response |
8 (53.3%) |
5 (33.3%) |
0.270 |
ACR: American College of Rheumatology; EULAR: European League Against Rheumatism; RA: rheumatoid arthritis.
Table 4: Effect of silibinin on different functional areas of HAQDI score of patients with active RA maintained on MTX.
|
Dress |
1.6±0.02 |
1.1±0.01*a |
1.7±0.01 |
1.4±0.04*b |
|
Arise |
1.3±0.03 |
0.8±0.01*a |
1.7±0.01 |
1.2±0.02*b |
|
Eat |
1.9±0.01 |
1.5±0.01*a |
1.8±0.04 |
1.6±0.01*a |
|
Walk |
1.9±0.03 |
1.6±0.04*a |
1.8±0.03 |
1.6±0.01*a |
|
Hygiene |
1.9±0.03 |
1.3±0.04*a |
1.6±0.01 |
1.4±0.03*a |
|
Reach |
1.9±0.03 |
1.2±0.01*a |
1.4±0.02 |
1.3±0.03*a |
|
Grip |
2.0±0.01 |
1.4±0.01*a |
1.6±0.01 |
1.3±0.01*a |
*significantly different compared to pre-treatment (p < 0.050); post-treatment values with different superscripts (a,b) within each parameter are significantly different (p < 0.050). HAQDI: health assessment questionnaire disability index; RA: rheumatoid arthritis.
The coadministration of silibinin with MTX produces a significant (p < 0.050) increase in Hb (10%) after 16 weeks compared to baseline values. This effect was significantly greater than that reported in the placebo group post-treatment [Table 5]. We observed a significant decrease in serum IL-8 levels in both silibinin and placebo groups (49% and 9%, respectively) compared to baseline values, but the effect of silibinin was significantly greater than in the placebo group. Moreover, serum IL-6 was significantly decreased in both groups (38% and 11%, respectively) at the end of the study. The effect of silibinin was significantly greater than that reported in placebo group after 16 weeks. Both treatment approaches produced a significant decrease in serum TNF-α (51% and 30%) after 16-weeks. However, the effect of silibinin was significantly greater than placebo. The coadministration of silibinin with MTX or placebo significantly elevates serum levels of IL-10 and IL-2 compared to baseline values; however, the effect of silibinin was significantly higher than that reported in the placebo group. Moreover, the administration of silibinin with MTX produces a greater decrease in anti-CCP antibody levels, which was significantly different compared to the placebo group post-treatment.
Table 5: Effect of silibinin on hemoglobin level and biomarkers of inflammation in patients with active RA maintained on methotrexate.
|
Hb, g/dL |
11.7±0.08 |
12.8±0.04*a |
11.6±0.1 |
11.9±0.1b |
|
IL-8, pg/ml |
182±3.0 |
92.3±10.0*a |
148.6±9.5 |
135.4±8.7b |
|
IL-6, pg/ml |
130±3.6 |
80.3±3.8*a |
130±4.3 |
116±2.7*b |
|
TNF-a, pg/ml |
16.5±0.7 |
8.1±0.8*a |
16.9±0.7 |
13.1±0.8*b |
|
IL-10, pg/ml |
1.4±0.1 |
7.5±0.5*a |
1.6±0.1 |
2.5±0.2*b |
|
IL-2, pg/ml |
1.5±0.2 |
12.5±1.2*a |
1.5±0.1 |
2.9±0.3*b |
*significantly different compared to pre-treatment (p < 0.050); post-treatment values with different superscripts (a,b) within each parameter are significantly different (p < 0.050). Hb: hemoglobin, IL: interleukin; TNF-α: tumor necrosis factor alpha; CCP: anti-cyclic citrullinated peptide.
Discussion
Apart from functioning as antioxidants, polyphenolic compounds have well-recognized anti-inflammatory properties.21 Based on evidence suggesting the anti-inflammatory activities of silibinin,13,22 we evaluated the adjuvant use of silibinin with MTX, and the effect on the clinical and biochemical markers of RA. In this pilot clinical study, we showed for the first time that coadministration of silibinin with MTX in the treatment of active RA may increase the efficacy of the latter to improve both the clinical and biochemical markers of the disease. The reported improvement in some clinical scores and attenuation of inflammatory cytokines release were consistent with the previously reported anti-inflammatory activities of many polyphenols in vitro and in vivo.23,24
Several inflammatory cytokines and pathogenic T-cells, such as IL-17-producing T-cells, are implicated in joint destruction,25,26 a major symptom of RA. Recently, we reported the antiarthritic effect of silibinin, when used alone or as an adjuvant with MTX, by decreasing the production of TNF-α, IL-1b and IL-8 in a rat model of zymosan-induced arthritis.12 The outcome of this animal study encouraged our efforts toward performing the present pilot clinical study. Our data suggested that silibinin may inhibit tissue destruction caused by excessive production and release of destructive inflammatory mediators within the inflamed joints. These results are in tune with previous reports, where silibinin decreases expression of the pro-inflammatory cytokine that may be attributed to various mechanisms including suppression of reactive oxygen species (ROS) generation.11,27,28 In our study, silibinin enhanced the antiarthritic activity of MTX, revealed as a significantly higher improvement in the biochemical markers and clinical scores compared to using MTX alone. Although the effect of silibinin alone was not evaluated due to ethical considerations, its concomitant use with other antiarthritic agents may show promising results, and future studies with larger patients sample and longer duration are needed to elucidate its effective role in this respect.
Silibinin is known to hinder the inflammatory process by inhibiting neutrophil migration; it also inhibits the formation of leukotrienes (by inhibiting 5-lipoxigenase pathway) and the release of histamine from basophils.29 Moreover, silibinin attenuates the production of edema and granulation tissue in experimental animal models of chronic inflammation and is suggested as a potential treatment for chronic inflammatory disorders.30 Because of the heterogeneous nature of RA, there is no single disease activity variable shown to be accurate or valid when used alone for diagnosis or treatment follow-up. Therefore, the ACR recommended the use of disease activity indices that include multiple variables (e.g., DAS28, HAQDI, or SDAI) for the accurate measurement of RA severity and treatment follow-up.31
In our study, coadministration of silibinin with MTX showed different outcomes in the disease activity indices. There was a significant improvement in DAS28 scores after 16 weeks compared to MTX plus placebo. Meanwhile, the effect on the SDAI scores compared with placebo was not significant. The difference in the results of these scores and the effect of silibinin depend on many factors that are variably modified in the silibinin-treated group, including the swollen and tender joint counts, visual analog scale, and the ESR. The anti-inflammatory role of silibinin in RA patients may be due to inhibition of cytokines production including TNF-α. This effect supports other previously reported data regarding use of natural products in RA or other inflammatory conditions.32–34 In this study, silibinin improves the capacity of MTX to decrease the production of many inflammatory markers including TNF-α and anti-CCP antibodies. This was also in agreement with other reports that address the role of natural products in RA treatment, where their combination with MTX was evaluated with respect to efficacy and safety of the later in prospective controlled trials.35,36 Moreover, supplements from natural sources could be of value in decreasing the adverse effects of conventional drugs used for the treatment of RA, and may improve cost and patient compliance.37 However, the extent of changes in the inflammatory markers was not associated with improvements in all clinical indices. This behavior seems to be common when using drugs that target TNF-α during RA treatment,38 and the study limitations mostly influence the association between the biochemical and clinical outcomes of these treatment approaches.
The modest benefits of silibinin reported in this pilot study, which included Iraqi patients with active RA, may be attributed to its pleiotropic effects, which interfere with many pathophysiological processes of RA including immunomodulatory, anti-inflammatory, and antioxidant activities. Meanwhile, other studies reported the dose-dependent antioxidant and anti-inflammatory activities of silibinin, and most of these effects were produced by using high daily doses (120 mg twice daily), comparable to that used in our study.39 Moreover, the limitations of small sample size and short duration should be considered when trying to explain the variable clinical and biochemical outcomes of this study, and a larger sample and longer clinical trial duration is highly recommended.
Conclusion
Silibinin may improve the effects of MTX on certain biochemical and clinical markers of patients with active RA.
Disclosure
The authors declared no conflict of interest. The project was funded by the University of Baghdad.
Acknowledgements
The authors thank the medical staff of Baghdad Teaching Hospital, Baghdad and Al-Sader Teaching Hospital, Al-Najaf for technical assistance.
references
- 1. Van-Assche T, Huygelen V, Crabtree MJ, Antoniades C. Gene therapy targeting inflammation in atherosclerosis. Curr Pharm Des 2011 Dec;17(37):4210-4223.
- 2. Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol Ther 2013 Aug;139(2):189-212.
- 3. Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med 2006 Aug;355(7):704-712.
- 4. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006 May;295(19):2275-2285.
- 5. Cachofeiro V, Miana M, de las Heras N, Beatriz Martin-Fernandez B, Sandra Ballesteros S, Balfagon G, et al, editors. Inflammation: a link between hypertension and atherosclerosis. Current Hypertension Reviews; 2009. p. 40-48 .
- 6. Lopes F, Coelho FM, Costa VV, Vieira EL, Sousa LP, Silva TA, et al. Resolution of neutrophilic inflammation by H2O2 in antigen-induced arthritis. Arthritis Rheum 2011 Sep;63(9):2651-2660.
- 7. Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys 2014 Oct;559:91-99.
- 8. Mossalayi MD, Rambert J, Renouf E, Micouleau M, Mérillon JM. Grape polyphenols and propolis mixture inhibits inflammatory mediator release from human leukocytes and reduces clinical scores in experimental arthritis. Phytomedicine 2014 Feb;21(3):290-297.
- 9. Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr Cancer Ther 2007 Jun;6(2):146-157.
- 10. Deep G, Agarwal R. Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr Cancer Ther 2007 Jun;6(2):130-145.
- 11. Gupta OP, Sing S, Bani S, Sharma N, Malhotra S, Gupta BD, et al. Anti-inflammatory and anti-arthritic activities of silymarin acting through inhibition of 5-lipoxygenase. Phytomedicine 2000 Mar;7(1):21-24.
- 12. Mortada AH, Hussain SA. Silibinin improves the anti-arthritic activity of methotrexate in zymosan-induced arthritis in rats. Am J Pharmacol Sci 2014;2(3):47-51.
- 13. Hussain SA, Jassim NA, Numan IT, Al-Khalifa II, Abdullah TA. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. A comparative study with piroxicam and meloxicam. Saudi Med J 2009 Jan;30(1):98-103.
- 14. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988 Mar;31(3):315-324.
- 15. Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995 Jan;38(1):44-48.
- 16. Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003 Feb;42(2):244-257.
- 17. Ghosh A, Ghosh B, Pain S, Pande A, Saha S, Banerjee A, et al. Comparison between DAS28, CDAI and HAQ-DI as tools to monitor early rheumatoid arthritis patients in eastern India. Indian J Rheumatol 2011;6(3):116-122 .
- 18. Li T, Wells G, Westhovens R, Tugwell P. Validation of a simple activity participation measure for rheumatoid arthritis clinical trials. Rheumatology (Oxford) 2009 Feb;48(2):170-175.
- 19. Jou JM, Lewis SM, Briggs C, Lee SH, De La Salle B, McFadden S; International Council for Standardization in Haematology. ICSH review of the measurement of the erythocyte sedimentation rate. Int J Lab Hematol 2011 Apr;33(2):125-132.
- 20. Balasubramaniam P, Malathi A, Viswanathan C. A simple technique for hemoglobin estimation to screen for anaemia. Indian J Physiol Pharmacol 1992 Jul;36(3):213-214.
- 21. Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1998 Nov;56(11):317-333.
- 22. Juma’a KM, Ahmed ZA, Numan IT, Hussain SA. Dose-dependent anti-inflammatory effect of silymarin in experimental animal model of chronic inflammation. Afr J Pharm Pharmacol 2009;3(5):242-247.
- 23. Natarajan V, Madhan B, Tiku ML. Intra-articular injections of polyphenols protect articular cartilage from inflammation-induced degradation: Suggesting a potential role in cartilage therapeutics. PLoS One 2015;10(6):e0127165.
- 24. Yun HJ, Yoo WH, Han MK, Lee YR, Kim JS, Lee SI. Epigallocatechin-3-gallate suppresses TNF-alpha -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblasts. Rheumatol Int 2008 Nov;29(1):23-29.
- 25. Ju JH, Cho ML, Moon YM, Oh HJ, Park JS, Jhun JY, et al. IL-23 induces receptor activator of NF-kappaB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J Immunol 2008 Jul;181(2):1507-1518.
- 26. Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 2006 Nov;203(12):2673-2682.
- 27. Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology 2007 May;132(5):1925-1936.
- 28. Ashkavand Z, Malekinejad H, Amniattalab A, Rezaei-Golmisheh A, Vishwanath BS. Silymarin potentiates the anti-inflammatory effects of Celecoxib on chemically induced osteoarthritis in rats. Phytomedicine 2012 Oct;19(13):1200-1205.
- 29. Bhattacharya S. Phytotherapeutic properties of milk thistle seeds: An overview. J Adv Pharmacy Edu Res 2011;1:69-79.
- 30. Aziz TA, Marouf BH, Ahmed ZA, Hussain SA. Anti-inflammatory activity of silibinin in animal models of chronic inflammation. Am J Pharmacol Sci 2014;2(1):7-11.
- 31. Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012 May;64(5):640-647.
- 32. Liu XD, Zhang JL, Ye LH. [Effects of wenhua juanbi recipe on TNF-alpha and IL-1beta in peripheral blood of rheumatoid arthritis patients]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009 Sep;29(9):787-790.
- 33. Chen ZW, Sun J, Li YM, Chen YQ. [Efficacy of Shenshi Qianghuo Dihuang Decoction in rheumatoid arthritis: a randomized controlled trial]. Zhong Xi Yi Jie He Xue Bao 2010 Jan;8(1):35-39.
- 34. Afshariani R, Farhadi P, Ghaffarpasand F, Roozbeh J. Effectiveness of topical curcumin for treatment of mastitis in breastfeeding women: a randomized, double-blind, placebo-controlled clinical trial. Oman Med J 2014 Sep;29(5):330-334.
- 35. Kogure T. Recent clinical applications of Japanese oriental (Kampo) medicine for rheumatoid arthritis: search for Kampo responder. Altern Ther Health Med 2013 Nov-Dec;19(6):50-52.
- 36. Kogure T, Tatsumi T, Sato H, Oku Y, Kishi D, Ito T. Traditional herbal medicines (Kampo) for patients with rheumatoid arthritis receiving concomitant methotrexate: a preliminary study. Altern Ther Health Med 2010 Jan-Feb;16(1):46-51.
- 37. Jie HY, Wu QF, Ding ZX. [Clinical study of Biqi Capsule combined with methotrexate for treatment of rheumatoid arthritis]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012 Feb;32(2):195-198.
- 38. Lv Q, Yin Y, Li X, Shan G, Wu X, Liang D, et al. The status of rheumatoid factor and anti-cyclic citrullinated peptide antibody are not associated with the effect of anti-TNFa agent treatment in patients with rheumatoid arthritis: a meta-analysis. PLoS One 2014;9(2):e89442.
- 39. Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol 1993 Sep;31(9):456-460.