|
INTRODUCTION
Breast cancers showing human epidermal receptor protein-2 (HER2/neu) alterations are critical to identify because it is vital to patient care following the approval of trastuzumab as the first therapy to target the HER2/neu oncoprotein. Also, HER2/neu expression has an independent adverse prognostic and predictive marker for invasive breast cancer.1-5 Therefore, analysis of the HER2/neu status of breast cancer specimen is assuming increasing clinical relevance.
The HER-2/neu oncogene encodes a 185 kDa transmembrane protein and is expressed at low levels in a variety of normal epithelia, including breast duct epithelium,5 and is amplified and over expressed in 20-30% of invasive breast cancers.6,7 The membrane localization of the protein forms the basis of immunohistochemistry (IHC), which is the most commonly used method of testing of HER2/neu over expression.1-5
In approximately 90% of the cases, protein over expression reflects underlying amplification of the HER2/neu gene located on chromosome 17 (17q21).8 In about 3% of the cases, over expression of HER2/neu can occur in the absence of gene amplification giving rise to false positive results on IHC and negative by Fluorescent in situ hybridization (FISH).9 It has been suggested that some of these IHC “false positive” results may be in part due to increased copy number of chromosome 17,10-13 resulting in an increased HER2/neu protein expression.14 False positive results are a significant problem where IHC is exclusively used to test for HER2/neu protein over expression. These are mostly confined to the group of 2+ by IHC using Hercep test and these patients are found not responding to the targeted therapy.15 In contrast, there is usually a high level of correlation between IHC 3+ staining and amplification detected by FISH analysis.16,17
The purpose of this study is to evaluate the factors contributing to false positive 3+ results by Hercep test kit IHC and subsequently not amplified for the HER-2/neu gene by Fluorescence in situ hybridization (FISH).
METHODS
In a retrospective five year study, out of the consecutive 164 cases of invasive breast cancers, all the 58 cases which were reported as 3+ for HER2 immunostaining by Hercep test at King Khalid University Hospital were reviewed by two pathologists. Among these, 26 equivocal cases were assorted and sent for FISH analysis for confirmation of HER2/neu gene amplification. 18 cases were selected for the study, which were reported originally as 3+ and turned out to be FISH negative. In each of these cases, HER2/neu IHC was repeated using Hercep Test, from the same tissue block used for the original IHC study. The current IHC slides were assessed, and the contributing factors, i.e. the technical error (i.e staining of blood vessels or benign ducts) and the interpretation errors were evaluated.
HER2/neu IHC analysis was performed on 4 micrometer sections of formalin fixed paraffin embedded tissue. Sections were stained using Hercep test kit (clone CB11) according to the manufactures instruction and the results were interpreted as follows using the original FDA and new ASCO/CAP guideline recommendations.14
Scoring was done on a 0-3 scale. Positive (3+) was defined as strong complete membranous staining in more than 30% of the tumor cell population. Borderline (2+) was defined as moderate membranous staining in more than 10% of tumor cells. While negative (1+) was defined as either weak or barely perceptible membranous staining in more than 10% of the tumor cells and zero was completely negative staining or membranous staining in less than 10% of the tumor cells. Scores of 0 and 1+ were considered as negative for Her-2/Neu expression, 3+ as immune-positive while 2+ were weakly or borderline positive. Positive and negative controls were included in each batch. Hercep test IHC results on all slides, original and repeat were scored by two pathologists.
FISH analysis was performed at Kassel Klinikum Laboratory (authorised laboratory in Germany) for HER-2/neu gene amplification. HER-2/neu gene amplification was determined by FISH according to Path Vysion (Vysis, Inc) in paraffin embedded tissue sections as a ratio of HER-2/neu gene copies-to chromosome 17 centromere copies. The HER2 gene was considered unamplified when the ratio was <2.0 and amplified when the ratio was >2.0. Cases with >2.7 chromosome 17 per nucleus on average were considered as polysomy.
RESULTS
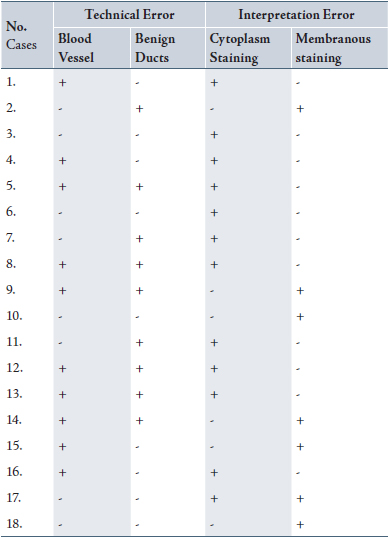
Five of the 18 cases were purely interpretation errors in the original pathology report and the remaining were the combination of technical and interpretation errors, (Table 1). In 61.2% (12/18) of cases, the strong cytoplasmic staining and in 38.8% (7/18) of cases, the strong granular membranous staining were misinterpreted as 3+ IHC positivity. 50% and 55.5% of the cases respectively showed benign breast duct and blood vessel wall positivity upon the review of original and current immunostained slides.
Table 1: Histopathological factors contributing to false positive results in Her2-neu 3+ IHC
DISCUSSION
In the present study, upon a review of the HER2/neu (3+) immunostains according to the manufacturers guidelines and FDA approved scoring system, 18 of 58 cases (3+) immunostains were re-classified as HER2/neu negative invasive breast cancers. This study attempted to analyze various technical or interpretative deviations from the prescribed methods leading to the false positive results.
The reasons for these false positive results varied. First and foremost, it was found that when the Hercep test assay was not used strictly according to the manufacturers FDA approved guidelines, had low specificity for the detection of the HER2/neu protein expression. Also, the technical methodological aspects of the assay contributed to false positive cases as seen in the current study. From experience, variation in type of fixative, length of tissue fixation details of tissue processing when used strictly according to manufacturers instructions can result in differences in the intensity of staining for HER2/neu in the tumor cells as well as in adjacent non neoplastic benign breast duct epithelium and blood vessels.
Furthermore, there can be error by the reporting pathologist in the interpretation of the results. Proper training of the pathologist to strictly adhere to the new ASCO/CAP guidelines can minimize this problem.
The key determinant of the utility of a test for HER2/neu gene is whether it is predictive of a patient’s response to the targeted therapy, trastuzumab. Most patients with a beneficial clinical response to Herceptin had tumors, which were 3+ by IHC. Some recent studies also suggest that FISH may be a better predictor of response to Herceptin than IHC.18 Cases which are positive for HER-2 by fluorescent in situ hybridization may also benefit from treatment regardless of whether they express HER-2 at the 2+ or 3+ level.19
CONCLUSION
Overall, false positive results related to technical and interpretation errors can be minimized by properly educating the technologist and pathologist to perform high quality immunostains and to render an accurate diagnosis respectively. In addition, all equivocal cases of 3+ or 2+ should be referred for confirmation by FISH analysis. This is of utmost importance in therapeutic implications where confirmatory molecular testing is not included in the routine evalution protocol for HER2/neu in invasive breast cancer.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
|