|
Abstract
Objectives: This short study aims to determine the prevalence of various bacterial pathogens causing infections in the Aseer regions, and to also assess the distribution of Staphylococcus aureus in relation to different body sites as well as their in vitro antimicrobial susceptibility profile.
Methods: Clinical specimens (n=9831) from various infections diagnosed at Aseer Central Hospital (ACH) and Abha General Hospital (AGH), were analyzed bacteriologically. Confirmed S. aureus isolates (n=210) were tested against 44 antibacterial agents as per standard methods.
Results: Bacterial pathogens were recovered from 24.9% of the samples. The results revealed that Escherichia coli, Klebsiella pneumoniae, Enterococcus spp. and S. aureus to be the main etiological agents, while purulent exudates of wounds and abscesses were the main source of S. aureus. Out of the 210 S. aureus isolates; 77 (38.5%) were recovered from purulent exudates of wounds and abscesses of the examined patients and 53 (26.5%) were from high vaginal discharges, while other body sites exhibited different rates of S. aureus. On the other hand, 45% of the 210 S. aureus isolates were found to be multidrug resistant S. aureus (MRSA).
Conclusion: The results from this study revealed that Escherichia coli and staphylococci were the main etiological agents, while purulent exudates of wounds and abscesses were the main source of S. aureus. Also, a higher rate of MRSA was detected.
Keywords: Bacterial pathogens; Aseer region; Staphylococcus aureus; MRSA.
Introduction
A survey for infectious agents is one of the most important epidemiological tools to trace infectious disease and to predict disease patterns, as well as determining the distribution of agents according to body sites. Hospitalized patients are at unusually high risk of infection for various reasons, and tend to be more susceptible to infection because of their underlying disease conditions, but the risk is compounded by exposure to certain invasive bacteria. In immunocompromised patients, even non-pathogenic microorganisms are capable of causing disease. Furthermore, the hospital environment supports the acquisition of resistance to antibiotic agents due to drug resistant pathogens.1
Nosocomial infections cause substantial morbidity and mortality, prolong the hospital stay of affected patients, and increase direct patient-care costs.2 S. aureus is one of the most frequently isolated pathogens in clinical specimens.3 In the United States, approximately 60% of staphylococcal infections in the intensive care unit are now caused by MRSA.4 The objective of this study was to determine the prevalence of various bacterial pathogens causing infections in the Aseer regions and to determine the distribution of Staphylococcus aureus in relation to different body sites and infection types, as well as to examine their in vitro antimicrobial susceptibility profile.
Methods
This was a prospective study conducted between March and September 2004. Clinical specimens were collected and bacteriological data of 9831 specimens from various infections were analysed at Aseer Central Hospital (ACH) and Abha General Hospital (AGH), in Abha city, Aseer region, Kingdom of Saudi Arabia. Samples were cultured on a blood-agar medium. Growth colonies were identified on the basis of cultural, microscopic and biochemical tests as per standard protocols.5 The results obtained concerning; type of infection, sex, age, specimen's source and clinical diagnosis were examined using analytical packages in the SPSS software (Version 10).
The specimen were tested to determine samples confirmed of the presence of bacterial pathogens against 44 antimicrobial agents belonging to various structural types and different modes of actions. The antimicrobial susceptibility test was performed by the disk diffusion method using Muller-Hinton agar plates as per the National Committee for Clinical Laboratory standards.6 Standard antibiotics disks (Oxoid) were used, which were placed on the agar with flamed forceps or a single disk applicator and gently pressed down to ensure contact. The plates were then incubated immediately, or within 30 min. In cases of several growth colonies within a zone of inhibition; the strain was checked for purity and retested. Isolates were categorized as sensitive or resistant based on standardized zones of inhibition.
Results
The overall prevalence of bacterial pathogens among the 9631 clinical samples collected was found to be 24.9% (2402 of 9631). The main organisms detected were: Escherichia coli 790 (32.9%) and staphylococci 576 (24%). Of the 576 staphylococci, 366 (15.2%) were coagulase-negative Staphylococcus spp. and 210 (8.7%) were S. aureus. Other bacteria namely; Enterococcus spp., group B, Streptococcus, Klebsiella spp., Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter baumanii, Pantoea spp., Enterobacter spp., Citrobacter koseri and Proteus mirabilis were isolated with different frequencies. (Fig. 1)
The results obtained from this study also revealed that S. aureus accounted for 36.5% (n=210) of the total staphylococci isolates. Out of the 210 S. aureus isolates; 77 (38.5%) were recovered from purulent exudates of wounds and abscesses of the examined patients and 53 (26.5%) were from high vaginal discharges, while other body sites revealed different rates of S. aureus.
The types of infections surveyed in the present study were either nosocomial or community-acquired infections. The focus of this study was the nosocomial infections; thus of the 210 S. aureus isolates; 100 were isolated from hospital-acquired infections (nosocomial infections), 100 were isolated from community acquired infections and 10 were isolated from hospital environment namely; sinks, floor, walls and incubators.
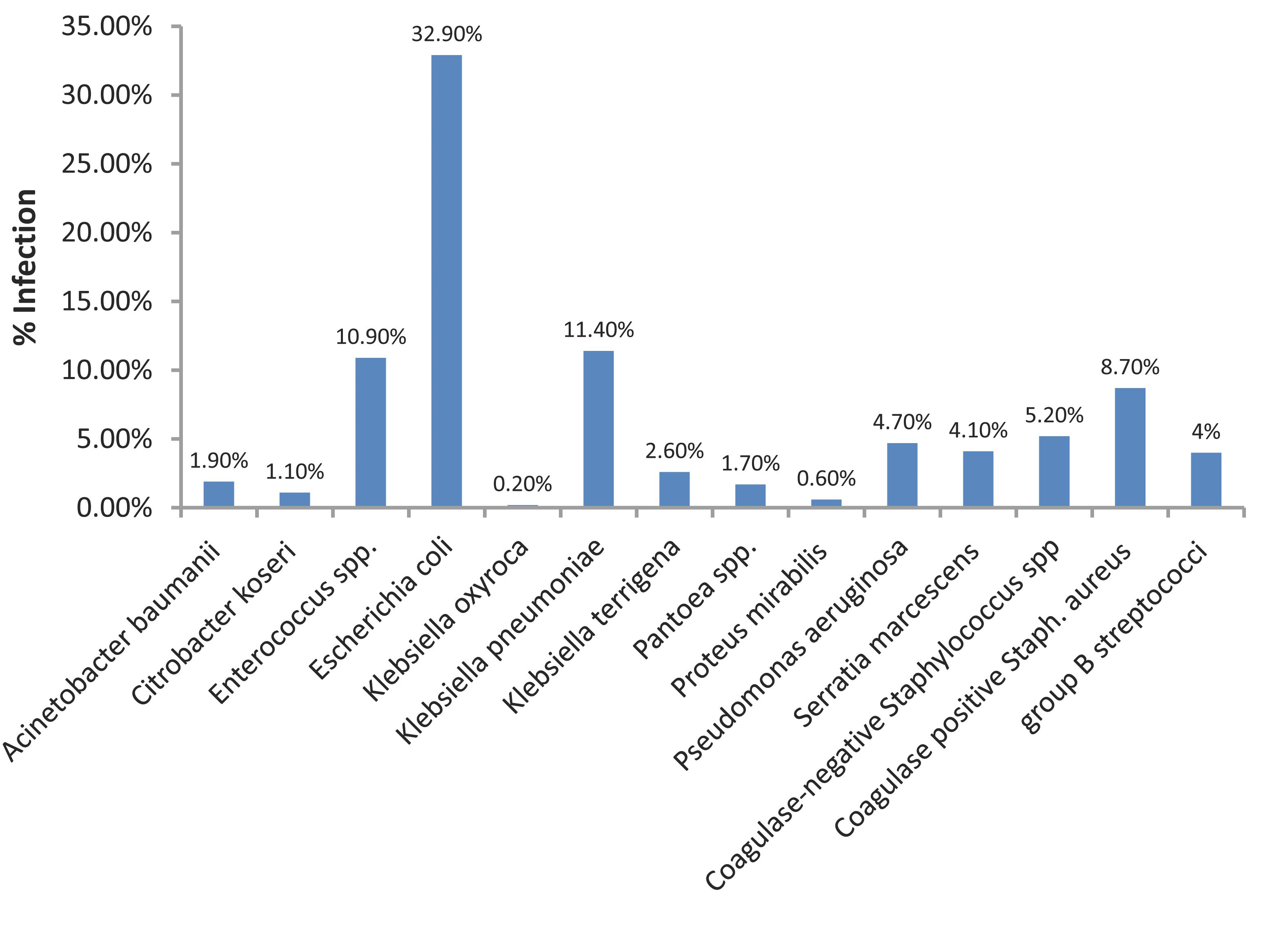
Figure 1: Recovery of various bacteria from patients in Aseer region in relation to Staphylococcus aureus.
Table 1: Percentage of resistant Staphylococcus aureus isolated from patients with a range of infections in Aseer Region. (n=210)
|
Antimicrobial agents
|
Resistant isolates (%)
|
|
1. Amikacin (30 µg)
|
23.5%
|
|
2. Amoxicillin (10 µg)
|
75.5%
|
|
3. Ampicillin (10 µg)
|
75.5%
|
|
4. Augmentin (30 µg)
|
38%
|
|
5. Aztreonam (30 µg)
|
100%
|
|
6. Bacitracin (10 IU)
|
17.5%
|
|
7. Carbenicillin (00 µg)
|
42%
|
|
8. Cefepime (30 µg)
|
31%
|
|
9. Cefotaxime (30 µg)
|
31%
|
|
10. Cefoxitin (30 µg)
|
33%
|
|
11. Ceftazidime (30 ug)
|
91%
|
|
12. Ceftriaxone (30 µg)
|
36.5%
|
|
13. Cephalothin (30 µg)
|
21.5%
|
|
14. Cephradine (30 ug)
|
34.5%
|
|
15. Chloramphenicol (30 µg)
|
1.5%
|
|
16. Ciprofloxacin (01 µg)
|
24%
|
|
17. Clindamycin (2 µg)
|
24.5%
|
|
18. Colistin (10 µg)
|
100%
|
|
19. Co-trimoxazole (25 µg)
|
27%
|
|
20. Doxycycline (30 µg)
|
15.5%
|
|
21. Erythromycin (15 µg)
|
21.5%
|
|
22. Fusidic acid (30 µg)
|
35%
|
|
23. Gentamicin (10 µg)
|
29%
|
|
24. Imipenem (10 µg)
|
22.5%
|
|
25. Mecillinam (33 µg)
|
100%
|
|
26. Methicillin (25 µg)
|
43.5%
|
|
27. Metronidazole (5 µg)
|
100%
|
|
28. Nalidixic acid (30 µg)
|
100%
|
|
29. Neomycin (30 µg)
|
33.5%
|
|
30. Netilmicin (30 µg)
|
22.5%
|
|
31. Nitrofurantoin (300 µg)
|
0%
|
|
32. Norfloxacin (10 µg)
|
25.5%
|
|
33. Novobiocin (30 µg)
|
0%
|
|
34. Oxacillin (10 µg)
|
43.5%
|
|
35. Oxytetracycline (30 µg)
|
24%
|
|
36. Penicillin G (10 IU)
|
87.5%
|
|
37. Piperacillin (100 µg)
|
54.5%
|
|
38. Polymyxin B (300 IU)
|
100%
|
|
39. Rifampicin (05 µg)
|
11%
|
|
40. Tetracycline (30 µg)
|
27.5%
|
|
41. Ticarcillin (75 µg)
|
36.5%
|
|
42. Tobramycin (10 µg)
|
29.5%
|
|
43. Trimethoprim-sulfamethoxazole (25 µg)
|
26%
|
|
44. Vancomycin (30 µg)
|
0%
|
The results of antibacterial susceptibility testing are shown in Table 1. The 210 S. aureus isolates were found to be resistant to aztreonam, colistin, mecillinam, metronidazole, polymyxin B and nalidixic acid, but all were found to be sensitive to vancomycin, nitrofurantoin and novobiocin. Moreover, various levels of resistance were recorded for the remaining antibiotics. Multidrug resistant S. aureus (MRSA) accounted for 45%; while 47% were multi-resistant MRSA, and 47% were non-multi-resistant MRSA.
Discussion
When comparing the results of the present study with previous two studies which had been conducted at AGH in 1996 and 1998,7 the prevalence of all positive cultures was found to be 30.7% in 1996 and 31.1% in 1998. S. aureus isolates in 1996 comprised 27.8% of all positive cultures while in 1998, the rate was 20.2% compared to 24.9% in the present study. The prevalence rates of bacterial pathogens and S. aureus in the present study have decreased compared to those found in the previous studies. These results can be attributed to the success of infection control system strategy aimed to prevent diseases and control of bacterial infection within the two hospitals.
Reports of S. aureus prevalence from many parts of the world, in contrast to the findings in the present study, were comparable. S. aureus infections have been documented, and were implicated as a causal organism in various diseases worldwide. In Middle Eastern countries, the prevalence of S. aureus was found to be 25% in Oman among infected babies,8 while the prevalence rate was 29% of infected wounds in Kingdom of Saudi Arabia.9 In some African countries such as Ethiopia, the prevalence of S. aureus was found to be 14.3% among all cases of bacteraemia.10
The proportion of MRSA reported in this study (45%) is higher compared to the reported percentage in other regions. In Latin America, MRSA was 34.9%, United States was 34.2%, Europe 26.3% and 5.7% in Canada.11 It was also observed that penicillin-resistant S. aureus detected in the present study was higher than compared to previously reported in Saudi Arabia.7,9 Many studies have indicated the increasing prevalence of MRSA in Saudi Arabia,12-14 recently, Bukharie reported increasing rates of MRSA from 20% by the end of 2004 to 59% at the end of 2008.15
Conclusion
Overall, a significant number of the studied specimens (75.1%) revealed negative growth for bacterial pathogens. Escherichia coli and staphylococci were the main etiological agents in the study, while purulent exudates of wounds and abscesses were the main source of S. aureus. Moreover, MRSA represented a higher rate (45%) compared to previous reports, a finding worth noting.
Acknowledgements
The technical help kindly provided by Mr. Hassan Ali Algarni and Abdulla Elshehrani is acknowledged. The authors reported no conflict of interest and no funding was received for this work.
References
1. Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 1993 Oct;6(4):428-442.2. Amin AN, Rehm SJ. Infections in hospitalized patients: what is happening and who can help? Cleve Clin J Med 2007 Aug;74(Suppl 4):S2-S5.
3. Tsering DC, Pal R, Kar S. Methicillin-resistant Staphylococcus aureus: prevalence and current susceptibility pattern in sikkim. J Glob Infect Dis 2011 Jan;3(1):9-13.
4. Rice LB. Antimicrobial resistance in gram-positive bacteria. Am J Infect Control 2006 Jun;34(5)(Suppl 1):S11-S19, discussion S64-S73.
5. Forbes B, Sahm DF, Weissfeld AS. (2002). Bailey and Scott's Diagnostic Microbiology, 11th Edition. Mosby: St. Louis.
6. National Committee for Clinical Laboratory Standards (NCCLS) (2002). Performance Standards for antimicrobial susceptibility testing. 8th Informational Supplement. M100 S12. National Committee for Clinical Laboratory Standards, 2002. Villanova, Pa.
7. Bilal NE, Gedebou M. Staphylococcus aureus as a paradigm of a persistent problem of bacterial multiple antibiotic resistance in Abha, Saudi Arabia. East Mediterr Health J 2000 Sep-Nov;6(5-6):948-954.
8. Rajab A, De Louvois J. Survey of infection in babies at the Khoula Hospital, Oman. Ann Trop Paediatr 1990 Mar;10(1):39-43.
9. Akbar DH, Mushtaq MA, El-Tahawi AT, Bahnasy AA. Staphylococcus aureus bacteremia. Saudi Med J 2000 Feb;21(2):171-174.
10. Asrat D, Amanuel YW. Prevalence and antibiotic susceptibility pattern of bacterial isolates from blood culture in Tikur Anbassa Hospital, Addis Ababa, Ethiopia. Ethiop Med J 2001 Apr;39(2):97-104.
11. Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, et al; SENTRY Partcipants Group. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis 2001l May;32(Suppl 2):S114-S132.
12. Madani TA. Epidemiology and clinical features of methicillin-resistant Staphylococcus aureus in the University Hospital, Jeddah, Saudi Arabia. Can J Infect Dis 2002 Jul;13(4):245-250.
13. Balkhy HH, Memish ZA, Almuneef MA, Cunningham GC, Francis C, Fong KC, et al. Methicillin-resistant Staphylococcus aureus: a 5-year review of surveillance data in a tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol 2007 Aug;28(8):976-982.
14. Bukhari EE, Al-Otaibi FE. Severe community-acquired infection caused by methicillin-resistant Staphylococcus aureus in Saudi Arabian children. Saudi Med J 2009 Dec;30(12):1595-1600.
15. Bukharie HA. Increasing threat of community-acquired methicillin-resistant Staphylococcus aureus. Am J Med Sci 2010 Nov;340(5):378-381.
|